Abstract
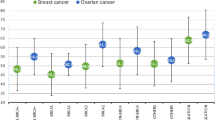
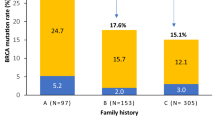
We have analyzed, by a combination of mutation and linkage analysis, the genetic basis of 22 breast cancer families in which at least 4 cases of either breast cancer diagnosed under the age of 60 or ovarian cancer had occurred. Chain-terminating mutations in BRCA1 were evidenced in 6 families, and posterior probabilities of <0.90 of being linked to BRCA1 in 3. The breast versus ovarian cancer ratio in these 9 families was approximately 2:1. Among the remaining 13 families, significant linkage to markers flanking BRCA2 was established in the admixture test with a maximum multipoint lod score of 3.38, but there was no statistical evidence for genetic heterogeneity. The breast:ovarian cancer ratio in these families was 7:1, suggesting BRCA2 confers a much lower risk for ovarian cancer than does BRCA1. These results suggest that BRCA2 will explain a significant proportion of hereditary breast cancer in the Netherlands, and, together with BRCA1, account for the majority of all high-risk families.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lynch HT, Marcus JM, Watson P, Conway T, Fitzsimmons ML, Lynch JF: Genetic epidemiology of breast cancer; in Lynch HT, Hirayama T: Genetic Epidemiology of Cancer. Boca Raton, CRC Press, 1989.
Hall J, Lee M, Newman B, Morrow J, Anderson L, Huey B, King M: Linkage of early-onset familial breast cancer to chromosome 17q21. Science 1990;250:1684–1689
Easton D, Bishop D, Ford D, Crockford G, the Breast Cancer Linkage Consortium: Genetic linkage analysis in familial breast and ovarian cancer: Results from 214 families. Am J Hum Genet 1993;52:678–701
Narod S, Feunten J, Lynch H, Watson P, Conway T, Kynch J, Lenoir G: Familial breastovarian cancer locus on chromosome 17q12q23. Lancet 1991;338:82–83
Ford D, Easton D, Bishop D, Narod S, Goldgar D, the Breast Cancer Linkage Consortium: Risks of cancer in BRCAl-mutation carriers. Lancet 1994,343:692–695.
Narod S, Ford D, Devilee P, Barkardottir R, Lynch H, Smith S, Ponder B, Weber B, Garber J, Birch J, Cornells R, Kelsell D, Spurr N, Smyth E, Haites N, Sobol H, Bignon Y, Chang-Claude J, Hamann U, Lindblom A, Borg A, Piver M, Gallion H, Struewing J, Whittemore A, Tonin P, Goldgar D, Easton D, the Breast Cancer Linkage Consortium: An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Am J Hum Genet 1995;56:254–264
Miki Y, Swensen J, Shattuck-Eidens D, Futreal P, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett L, Ding W, Bell R, Rosenthal J, Hussey C, Tan T, McClure M, Frye C, Hattier T, Phelps R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, Stone S, Bayer S, Wray C, Bogden R, Dayananth P, Ward J, Tonin P, Narod S, Bristow P, Norris F, Helvering L, Morrison P, Rosteck P, Lai M, Barrett J, Lewis C, Neuhausen S, Cannon-Albright L, Goldgar R, Kamb A, Wiseman R, Skolnick M: A strong candidate for the breast and ovarian cancer susceptibility gene BRCAl. Science 1994;266:66–71
Shattuck-Eidens D, McClure M, Simard J, Labrie F, Narod S, Couch F, Hoskins K, Weber B, Castilla L, Erdos M, Brody L, Friedman L, Ostermeyer E, Szabo C, King M, Jhanwar S, Offit K, Norton L, Gilewski T, Lubin M, Osborne M, Black D, Boyd M, Steel M, Ingles S, Haile R, Lindblom A, Olsson H, Borg A, Bishop T, Solomon E, Radice P, Spatti G, Gayther S, Ponder B, Warren W, Stratton M, Liu Q, Fujimura F, Lewis C, Skolnick M, Goldgar D: A collaborative survey of 80 mutations in the BRCAl breast and ovarian cancer susceptibility gene: Implications for presymptomatic testing and screening. JAMA 1995;273:535–541
Hogervorst F, Cornells R, Bout M, Van Vliet M, Oosterwijk J, Olmer R, Bakker B, Klijn J, Vasen H, Meijers-Heijboer H, Menko F, Cornelisse C, Den Dunnen J, Devilee P, Van Ommen G: Rapid detection of BRCAl mutations by the protein truncation test. Nat Genet 1995;10:208–212
Stratton M, Ford D, Neuhausen S, Seal S, Wooster R, Friedman L, King M-C, Egilsson V, Devilee P, McManus R, Daly P, Smyth E, Ponder B, Peto J, Cannon-Albright L, Easton D, Goldgar D: Familial male breast cancer is not linked to the BRCA1 locus on chromosome 17q. Nature Genet 1994;7:103–107
Wooster R, Neuhausen S, Mangion J, Quirk Y, Ford D, Collins N, Nguyen K, Seal S, Tran T, Avenll D, Fields P, Marshall G, Narod S, Lenoir G, Lynch H, Feunteun J, Devilee P, Cornehsse C, Menko F, Daly P, Ormiston W, McManus R, Pye C, Lewis C, Cannon-Albright L, Peto J, Ponder B, Skolnick M, Easton D, Goldgar D, Stratton M: Localization of a breast cancer susceptibility gene BRCA2 to chromosome 13ql2. Science 1994;265:2088–2090
Devilee P, Cornells R, Bootsma A, Bardoel A, Van Vhet M, Van Leeuwen I, De Klein A, Lindhout D, Vasen H, Cornelisse C, Meera Khan P: Linkage to markers for the chromosome region 17ql2-q21 in 13 Dutch breast cancer kindreds. Am J Hum Genet 1993;52:730–735
Cornelis R. Vasen H, Meijers-Heijboer H, Ford D, Van Vliet M, Van Tilborg A, Cleton F, Klijn J, Menko F, Meera Khan P, Cornelisse C, Devilee P: Age at diagnosis as an indicator of eligibility for BRCA1 DNA-testing in familial breast cancer. Hum Genet 1995;95:539–544
Isola J, de Vries S, Chu L, Ghazvini S, Waldman F: Analysis of changes in DNA sequence copy number by comparative genomic hybridization in archival paraffin-embedded tumor samples. Am J Pathol 1994;145:1301–1308
Lathrop GM, Lalouel JM, Juher C, Ott J: Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sei USA 1984;81:3443–3446
Terwilliger JD, Ott J: Handbook of Human Genetic Linkage. Baltimore, The Johns Hopkins University Press, 1994.
Friedman L. Ostermeyer E, Szabo C, Dowd P, Lynch E, Rowell S, King M: Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet 1994:8:399–404.
Narod S, Ford D, Devilee P, Bakardottir RB, Eyfjörd J, Lenoir G, Serova O, Easton D, Goldgar D: Genetic heterogeneity of breast-ovarian cancer revisited. Am J Hum Genet 1995;57:957–958
Schutte M, Da Costa L, Hahn S, Moskaluk C, Hoque A, Rozenblum E, Weinstein C, Bittner M, Meitzer P, Trent J, Yeo C, Hruban R, Kern S: Identification by representational difference analysis of a homozygous deletion in pancreatic carcinoma that lies within the BRCA2 region. Proc Natl Acad Sei USA 1995;92:5950–5954
Simard J, Tonin P, Durocher F, Morgan K, Rommens J, Gingras S, Samson C, Leblanc J, Belanger C, Dion F, Liu Q, Skolnick M, Goldgar D, Shattuck-Eidens D, Labrie F, Narod S: Common origins of BRCA 1 mutations in Canadian breast and ovarian cancer families. Nat Genet 1994;8:392–398
Struewing J, Abeliovich D, Peretz T, Avishai N, Kaback M, Collins F, Brody L: The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet 1995;11:198–200
Johannsson O, Ostermeyer EA, Håkansson S, Friedman LS, Johansson U, Sellberg C, Brondum-Nielsen K, Sele V, Olsson H, King MC, Borg A: Founding BRCA 1 mutations in hereditary breast and ovarian cancer in southern Sweden. Am J Hum Genet 1996;58:441–450
Neuhausen SL, Mazoyer S, Friedman L, Stratton M, Offit K, Caligo A, Tomlinson G, Cannon-Albright L, Bishop T, Kelsell D, Solomon E, Weber B, Couch F, Struewing J, Tonin P, Durocher F, Narod S, Skolnick MH, Lenoir G, Serova O, Ponder BAJ, Stoppa-Lyonnet D, Easton D, King M-C, Goldgar DE: Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: results of an international study. Am J Hum Genet 1996;58:271–280
Gayther SA, Warren W, Mazoyer S, Russell PA, Harrington PA, Chiano M, Seal S, Hamoudi R, Van Rensburg EJ, Dunning AM, Love R, Evans G, Easton D, Clayton D, Stratton MR, Ponder BAJ: Germline mutations of the BRCA 1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet 1995;11:428–433
Serova O, Montagna M, Torchard D, Narod S, Tonin P, Sylla B, Lynch H, Feunteun J, Lenoir G: A high incidence of BRCA 1 mutations in 20 breast-ovarian cancer families. Am J Hum Genet 1996;58:42–51
Acknowledgements
The authors thank Mrs. I. van Leeuwen for blood sampling, and Dr. D.F. Easton for statistical support. This work was supported by a grant from the Dutch Cancer Society and is part of ongoing studies within the Breast Cancer Linkage Consortium, supported by BIOMEDl-grant PL920890.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peelen, T., Cornelis, R.S., van Vliet, M. et al. The Majority of 22 Dutch High-Risk Breast Cancer Families Are due to Either BRCA1 or BRCA2. Eur J Hum Genet 4, 225–230 (1996). https://doi.org/10.1159/000472203
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1159/000472203
Key Words
This article is cited by
-
The c.156_157insAlu BRCA2 rearrangement accounts for more than one-fourth of deleterious BRCA mutations in northern/central Portugal
Breast Cancer Research and Treatment (2009)
-
G1738R is a BRCA1 founder mutation in Greek breast/ovarian cancer patients: evaluation of its pathogenicity and inferences on its genealogical history
Breast Cancer Research and Treatment (2008)
-
BRCA1 genomic deletions are major founder mutations in Dutch breast cancer patients
Nature Genetics (1997)