Abstract
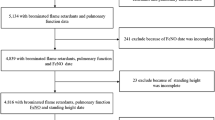
WITH reference to Prof. Porter's note in NATURE of June 13 (p. 151) on the odour of bromine detected on opening a sealed tube of radium bromide, it appears that the minimum quantity of bromine that is detectable by smell is between the orders 10-8to 10-10 grams per cubic centimetre of air. This result has been obtained by the progressive dilution of a definite volume of bromine vapour. It may be mentioned that the vapour of bromine is just detectable by its odour at the temperature of liquid air.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
EGERTON, A. Decomposition of Radium Bromide. Nature 76, 174 (1907). https://doi.org/10.1038/076174d0
Issue date:
DOI: https://doi.org/10.1038/076174d0