Abstract
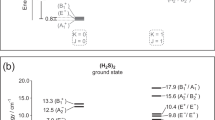
THE further examination of the secondary spectrum of hydrogen has led to a number of interesting discoveries since my last publication (Roy. Soc. Proc. A, vol. 111, p. 714, 1926). It appears that the green and red bands have the same electron jump but correspond to different sets of vibrational transitions, those for the green bands being 0→0, 1→1, 2→2, 3→3, and 4→4, and those for the red bands 1→0, 2→1, 3→2, 4→3, 5→3, and 6→5. In addition, there are five other less well developed sets of bands with the same electron jump, two in the infra-red with vibrational transitions 2→0, 3→1, 4→2 and 3→0, 4→1 respectively, and three on the violet side of the green with the respective sets of vibrational transitions 0→1, 1→2, 2→3, 3→4 and 0→2, 1→3 and 0→3. There is an intercombination between the lines of all the above bands and indications of a further combination in the members of the P R branches, which, however, are less well developed than the Q branches. The second differences of the Q branches form a square array with a common vertical difference = 2.4 and a common horizontal difference = 2.8 wave number.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RICHARDSON, O. The Secondary Hydrogen Spectrum. Nature 118, 116 (1926). https://doi.org/10.1038/118116a0
Issue date:
DOI: https://doi.org/10.1038/118116a0
This article is cited by
-
�ber das Molek�lspektrum des Wasserstoffs mit Wellenl�ngenmessungen von 3667 Linien zwischen ? 4861 (H ?) und 3314 �.-E
Zeitschrift f�r Physik (1929)