Abstract
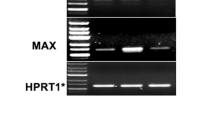
Enforced c-Myc expression promotes continuous, growth factor-independent, cell cycle progression and activates expression of the ornithine decarboxylase (ODC) gene and its promoter. c-Myc-responsiveness of murine ODC is mediated by two conserved c-Myc:Max E-boxes in ODC intron 1. c-Myc and ODC are both required for cell growth and their expression is sequentially induced in G0/G1 cells stimulated with mitogens, yet their expression is not modulated by the cell cycle in proliferating cells. Here we demonstrate that regulation of ODC and its promoter by Interleukin-3 (IL-3) in murine myeloid cells is mediated in part by c-Myc. c-Myc induced ODC through the same transcription start site as IL-3 and, in asynchronously growing cells, maximal activity of the ODC promoter required the intronic c-Myc binding sites. However, induction of ODC following IL-3 stimulation of quiescent cells is mediated by at least two pathways. The first phase of this response was independent of the intronic c-Myc:Max E-boxes and de novo protein synthesis. Sustained induction of the ODC promoter however required the c-Myc:Max binding sites and protein synthesis. Accumulation of c-Myc following stimulation of quiescent cells with IL-3 correlated with the delayed phase of the response. Consistent with a two pathway model of ODC regulation, inducible overexpression of dominant negative form of c-Myc (In373-Myc), which specifically inhibits the c-Myc-Max network, inhibited the delayed, but not immediate, induction of ODC promoter activity in response to IL-3. Dominant negative c-Myc protein also effectively suppressed induction of the endogenous ODC gene by IL-3. Therefore, c-Myc functions as a direct and required regulator of ODC. These results also suggest a model whereby c-Myc's role in regulating its targets may be to convert a transient, immediate-early, activation event into the persistent induction of gene expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Packham, G., Cleveland, J. Induction of ornithine decarboxylase by IL-3 is mediated by sequential c-Myc-independent and c-Myc-dependent pathways. Oncogene 15, 1219–1232 (1997). https://doi.org/10.1038/sj.onc.1201273
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/sj.onc.1201273
Keywords
This article is cited by
-
Arf tumor suppressor disrupts the oncogenic positive feedback loop including c-Myc and DDX5
Oncogene (2015)
-
In vivo – in vitro toxicogenomic comparison of TCDD-elicited gene expression in Hepa1c1c7 mouse hepatoma cells and C57BL/6 hepatic tissue
BMC Genomics (2006)
-
Bcl-2 is an apoptotic target suppressed by both c-Myc and E2F-1
Oncogene (2001)
-
Mechanisms of apoptosis by c-Myc
Oncogene (1999)
-
Tumor promoter-induced ornithine decarboxylase gene expression occurs independently of AP-1 activation
Oncogene (1999)