Abstract
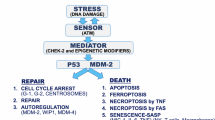
The tumor suppressor p53 is an inducer of cell cycle arrest and programmed cell death (apoptosis). The ability of p53 to induce cell cycle arrest is linked to its ability to induce transcription of genes such as the cyclin-dependent kinase inhibitor p21. However, the dependence of p53-mediated apoptosis on transcriptional activation remains controversial. Ectopic expression of a temperature-sensitive (ts) p53 allele induced expression of p53 target genes and elicited both G1 and G2/M cell cycle arrest upon shift to the permissive temperature. Ectopic expression of the same ts p53 allele with two additional point mutations (Gln22, Ser23) that abolish p53-transcriptional activation did not induce p53 target genes and G1 nor G2/M cell cycle arrest. In HCT116 colon carcinoma cells ectopic expression of wild type p53 does not elicit apoptosis whereas p53 mutant deficient in trans-activation induces apoptosis. The ability of wild type p53 to induce apoptosis is restored in HCT116 cells that are null for p21. However, the trans-activation deficient mutant of p53 is still more potent mediator of apoptosis than wild type p53 in the p21 null cells. Although the ability of Gln22,Ser23 to trans-activate p53 target genes is diminished, it retains the ability to repress Bcl-2 expression. Thus, we conclude that while ectopic expression of wild type p53 can induce both G1 and G2/M arrest, in a p21 dependent manner, without apoptosis, a p53 mutant defective in trans-activation elicits apoptosis without inducing cell cycle arrest. Further, the anti-apoptotic function of p53 is dependent on trans-activation and is linked to cell cycle arrest. The results strongly suggest that the trans-activation deficient mutant is a more potent inducer of apoptosis because it lost its anti-apoptotic function and retains its ability to repress pro-apoptotic genes such as Bcl-2. Taken together, the results imply that employing a trans-activation deficient p53 in gene therapy approaches or the use of drugs that convert mutant p53 to a trans-activation-independent mediator of apoptosis may be much more efficient therapeutic approaches than current approaches that employ wild type p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Attardi LD, Lowe SW, Brugarolas J and Jacks T. . 1996 EMBO J. 15: 3693–3701.
Bates S and Vousden KH. . 1999 Cell. Mol. Life Sci. 55: 28–37.
Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R and Weissberg P. . 1998 Science 282: 290–293.
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T and Hannon GJ. . 1995 Nature 377: 552–557.
Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR and Kley N. . 1995 Nature 377: 646–649.
Burns TF and El-Deiry WS. . 1999 J. Cell. Physiol. 181: 231–239.
Caelles C, Helmburg A and Karin M. . 1994 Nature 220–223.
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S and Reed JC. . 1998 Science 282: 1318–1321.
Chan TA, Hwang PM, Hermeking H, Kinzler KW and Vogelstein B. . 2000 Genes Dev. 14: 1584–1588.
Chao C, Saito S, Kang J, Anderson CW, Appella E and Xu Y. . 2000 EMBO J. 19: 4967–4975.
Deng C, Zhang P, Harper JW, Elledge SJ and Leder P. . 1995 Cell 82: 675–684.
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and Vogelstein B. . 1993 Cell 75: 817–825.
Gannon JV, Greaves R, Iggo R and Lane DP. . 1990 EMBO J. 9: 1595–1602.
Gottlieb TM and Oren M. . 1998 Semin. Cancer Biol. 8: 359–368.
Harn HJ, Ho LI, Liu CA, Liu GC, Lin FG, Lin JJ, Chang JY and Lee WH. . 1996 Histopathology 28: 317–323.
Harper JW, Adami GR, Wei N, Keyomarsi K and Elledge SJ. . 1993 Cell 75: 805–816.
Haupt Y, Maya R, Kazaz A and Oren M. . 1997 Nature 387: 296–299.
Isaacs WB, Carter BS and Ewing CM. . 1991 Cancer Res. 51: 4716–4720.
Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN and Hay N. . 1997 Genes Dev. 11: 701–713.
Levine AJ. . 1997 Cell 88: 323–331.
Lin J, Chen J, Elenbaas B and Levine J. . 1994 Genes Dev. 8: 1235–1246.
Michalovitz D, Halevy O and Oren M. . 1990 Cell 62: 671–680.
Miyashita T, Harigai M, Hanada M and Reed JC. . 1994a Cancer Res. 54: 3131–3135.
Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC. . 1994b Oncogene 9: 1799–1805.
Miyashita T and Reed JC. . 1995 Cell 80: 293–299.
Murphy M, Hinman A and Levine AJ. . 1996 Genes Dev. 10: 2971–2980.
Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, Roth JA, Deisseroth AB, Zhang WW, Kruzel E and Radinsky R. (1995). . Mol. Cell. Biol. 15: 3032–3040.
Polyak K, Waldman T, He TC, Kinzler KW and Vogelstein B. . 1996 Genes Dev. 10: 1945–1952.
Polyak K, Xia Y, Zweier JL, Kinzler KW and Vogelstein B. . 1997 Nature 389: 300–305.
Sabbatini P, Chiou S-K, Rao L and White E. . 1995 Mol. Cell. Biol. 15: 1060–1070.
Shen Y and Shenk T. . 1994 Proc. Nat. Acad. Sci. USA 91: 8940–8944.
Sinov RV and Haupt Y. . 1999 Oncogene 18: 6145–6157.
Wagner AJ, Kokontis JM and Hay N. . 1994 Genes Dev. 8: 2817–2830.
Wagner AJ, LeBeau MM, Diaz MO and Hay N. . 1992 Proc. Natl. Acad. Sci. USA 89: 3111–3115.
Wagner AJ, Small MB and Hay N. . 1993 Mol. Cell. Biol. 13: 2432–2440.
Wu GS, Burns TF, McDonald ER 3rd, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G and el-Deiry WS. . 1997 Nat. Genet. 17: 141–143.
Wu X, Bayle JH, Olson D and Levine AJ. . 1993 Genes Dev. 7: 1126–1132.
Yew PR, Liu X and Berk AJ. . 1993 Genes Dev. 8: 190–202.
Zhu J, Zhang S, Jiang J and Chen X. . 2000 J. Biol. Chem. 275: 39927–39934.
Zhu J, Zhou W, Jiang J and Chen X. . 1998 J. Biol. Chem. 273: 13030–13036.
Acknowledgements
We thank A Levine for the Gln22, Ser23 p53 cDNA and T Waldman and B Vogelstein for the isogenic cell lines HCT116 and HCT116 p21−/−. This work was supported by NIH grants CA-71874, AG-16927 (N Hay), CA-58073 and DK-41670 (S Liao).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kokontis, J., Wagner, A., O'Leary, M. et al. A transcriptional activation function of p53 is dispensable for and inhibitory of its apoptotic function. Oncogene 20, 659–668 (2001). https://doi.org/10.1038/sj.onc.1204139
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.onc.1204139
Keywords
This article is cited by
-
Gene delivery of cyclin-dependent kinase inhibitors p21 Waf1 and p27 Kip1 suppresses proliferation of MCF-7 breast cancer cells in vitro
Breast Cancer (2014)
-
p21 does not protect cancer cells from apoptosis induced by nongenotoxic p53 activation
Oncogene (2011)
-
Tid1 is a new regulator of p53 mitochondrial translocation and apoptosis in cancer
Oncogene (2010)
-
Cytoplasmic mutant p53 increases Bcl-2 expression in estrogen receptor-positive breast cancer cells
Apoptosis (2007)
-
Reversible inactivation of the transcriptional function of P53 protein by farnesylation
BMC Biotechnology (2006)