Abstract
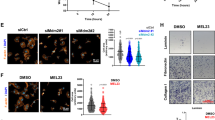
The transcription factor E2F1 functions as a key regulator for both cell-cycle progression and apoptosis. Mdm2, a major cellular regulator of the p53 tumor suppressor protein, is also closely involved in cell cycle and apoptosis. In addition to regulation of p53, Mdm2 has been reported to stimulate E2F1 transactivation by a mechanism that remains unclear. Here we examined how overexpression of Mdm2 alters E2F1/DP1 transactivation. Using a set of cell lines with differing p53 and Rb status we determined that Mdm2 induction of E2F1 transactivation was p53-dependent, resulting from release of repression by p53. While Mdm2 association with p53 was required to increase E2F1 transactivation, Mdm2 mediated degradation of p53 was not. p53 repression of E2F1 transactivation required a functional DNA binding and transactivation domain. Consistent with Mdm2 activation of E2F1 via an inhibition of p53 transactivation we demonstrate a concomitant reduction in p21 protein levels with Mdm2 overexpression. Furthermore, E2F1 repression by an Rb-phosphorylation mutant could not be reversed by Mdm2 overexpression. Mdm2 was also unable to enhance E2F1 transactivation in Mouse embryo fibroblasts lacking p21. Taken together, these results suggest that Mdm2 activation of E2F1 occurs through the repression of p53-dependent transcription of p21, a p53-target gene and cyclin dependent kinase inhibitor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD . 1997 J. Clin. Invest. 100: 2722–2728
Blattner C, Sparks A, Lane D . 1999 Mol. Cell. Biol. 19: 3704–3713
Boyd SD, Tsai KY, Jacks T . 2000 Nat. Cell. Biol. 2: 563–568
Cahilly-Snyder L, Yang-Feng T, Francke U, George DL . 1987 Som. Cell Mol. Gen. 13: 235–244
Chen C, Okayama H . 1987 Mol. Cell. Biol. 7: 2745–2752
Cress WD, Nevins JR . 1994 J. Virol. 68: 4213–4219
Dyson N . 1998 Genes Dev. 12: 2245–2262
Fakharzadeh SS, Trusko SP, George DL . 1991 EMBO J. 10: 1565–1569
Gartel AL, Serfas MS, Gartel M, Goufman E, Wu GS, El-Deiry WS, Tyner AL . 1996 Exp. Cell Res. 227: 171–181
Geyer RK, Yu ZK, Maki CG . 2000 Nat. Cell. Biol. 2: 569–573
Haines DS, Landers JE, Engle LJ, George DL . 1994 Mol. Cell. Biol. 14: 1171–1178
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ . 1993 Cell 75: 805–816
Haupt Y, Maya R, Oren M . 1997 Nature 387: 296–
Hiyama H, Iavarone A, Reeves SA . 1998 Oncogene 16: 1513–1523
Holmberg C, Helin K, Sehested M, Karlstrom O . 1998 Oncogene 17: 143–155
Hsieh JK, Chan FS, O'Connor DJ, Mittnacht S, Zhong S, Lu X . 1999 Mol. Cell 3: 181–193
Hsieh JK, Fredersdorf S, Kouzarides T, Martin K, Lu X . 1997 Genes Dev. 11: 1840–1852
Hunt KK, Deng J, Liu TJ, Wilson-Heiner M, Swisher SG, Clayman G, Hung MC . 1997 Cancer Res. 57: 4722–4726
Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W, Flores ER, Tsai KY, Jacks T, Vousden KH, Kaelin Jr WG . 2000 Nature 407: 645–648
Jackson M, Berberich SJ . 2000 Mol. Cell. Biol. 20: 1001–1007
Jackson MW, Berberich SJ . 1999 DNA Cell. Biol. 18: 693–700
Jackson MW, Lindstrom MS, Berberich SJ . 2001 J. Biol. Chem. 10: 10–
Johnson DG, Schwarz JK, Cress WD, Nevins JR . 1993 Nature 365: 349–352
Kubbutat MHG, Jones SN, Vousden KH . 1997 Nature 387: 299–
Lin J, Chen J, Elenbaas B, Levine AJ . 1994 Genes Dev. 8: 1235–1246
Lin J, Jin X, Page C, Sondak VK, Jiang G, Reynolds RK . 2000 Cancer Res. 60: 5895–5901
Mack DH, Vartikar J, Pipas JM, Lamininns LA . 1993 Nature 363: 281–283
Martin K, Trouche D, Hagemeier C, Sorensen TS, La Thangue NB, Kouzarides T . 1995 Nature 375: 691–694
Momand J, Wu HH, Dasgupta G . 2000 Gene 242: 15–29
Momand J, Zambetti GP, Olson DC, George D, Levine AJ . 1992 Cell 69: 1237–1245
Muller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K . 2001 Genes Dev. 15: 267–285
Nip J, Strom DK, Eischen CM, Cleveland JL, Zambetti GP, Hiebert SW . 2001 Oncogene 20: 910–920
Nip J, Strom DK, Fee BE, Zambetti G, Cleveland JL, Hiebert SW . 1997 Mol. Cell. Biol. 17: 1049–1056
O'Connor DJ, Lam EW, Griffin S, Zhong S, Leighton LC, Burbidge SA, Lu X . 1995 EMBO J. 14: 6184–6192
Phillips AC, Bates S, Ryan KM, Helin K, Vousden KH . 1997 Genes Dev. 11: 1853–1863
Qin XQ, Livingston DM, Kaelin Jr WG, Adams PD . 1994 Proc. Natl. Acad. Sci. USA 91: 10918–10922
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM . 1990 Cell 63: 1129–1136
Sherr CJ . 1998 Genes Dev. 12: 2984–2991
Stiewe T, Putzer BM . 2000 Nat. Genet. 26: 464–469
Wu X, Levine AJ . 1994 Proc. Natl. Acad. Sci. USA 91: 3602–3606
Xiao ZX, Chen J, Levine AJ, Modjtahedi N, Xing J, Sellers WR, Livingston DM . 1995 Nature 375: 694–698
Acknowledgements
We are indebted to the many colleagues who generously provided reagents, cell lines and plasmids. This work is supported by a NIH grant (CA64430) to SJ Berberich.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
This article is cited by
-
KMTase Set7/9 is a critical regulator of E2F1 activity upon genotoxic stress
Cell Death & Differentiation (2014)