Abstract
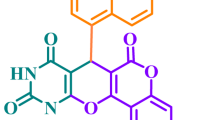
IN the course of an investigation of the structure of glucuronic acid, we have obtained results which clearly prove that this acid, like the related hexose d-glucose, possesses a pyranoid structure. Our starting material was bornyl-d-glucuronide (borneol glucuronic acid) isolated as the zinc salt from the urine of humans and dogs receiving small doses of borneol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jour. Chem. Soc., 123, 1356 ; 1923.
Jour. Chem. Soc., 350 ; 1926.
Jour. Chem. Soc., 258 ; 1931.
Ber. der Deutsch. chem. Gesell., 53, 17 ; 1920.
Jour. Chem. Soc., 127, 358 ; 1925.
Ber. der Deutsch. chem. Gesell., 30, 2584 ; 1897.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PRYDE, J., WILLIAMS, R. The Pyranoid Structure of Glucuronic Acid and of Theophylline Arabinoside. Nature 128, 187 (1931). https://doi.org/10.1038/128187a0
Issue date:
DOI: https://doi.org/10.1038/128187a0