Abstract
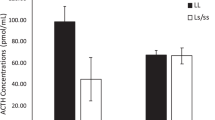
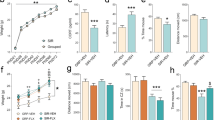
Previous research has shown that offspring of females with low cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA) concentrations are less likely to survive the first year of life than are offspring of females with high CSF 5-HIAA concentrations. In addition, studies of free-ranging rhesus macaque males have suggested that individuals with low CSF 5-HIAA concentrations suffer reduced reproductive success relative to their high serotonin counterparts. We examined CSF concentrations of the monoamine metabolites 5-HIAA and homovanillic acid (HVA), and plasma cortisol concentrations as predictors of first-time adult reproductive potential, maternal behavior, and overall social interactions in two groups of captive female rhesus macaques and their first offspring. Repeated CSF and blood samples were obtained from adult females in two social groups, and focal observations were performed for both new mothers and infants during the first month following parturition. We found that the reproductively aged nulliparous females who failed to give birth to their first offspring showed significantly lower CSF 5-HIAA concentrations than those females who gave birth. Among those females that gave birth to offspring, females with low CSF 5-HIAA concentrations and females with high plasma cortisol concentrations were overly protective and restrictive with their infants. CSF HVA concentration was not associated with reproductive output, social behavior, aggression, or mother–infant interactions in this sample of rhesus macaque females. We conclude that low CNS serotonin activity and high stress, measured by high plasma cortisol, are correlated with reduced reproductive success and patterns of high maternal restrictiveness in young adult female rhesus macaques.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ainsworth MDS, Blehar MC, Waters E, Wall S (1978). Patterns of Attachment: A Psychological Study of the Strange Situation. Lawrence Erlbaum Associates: Hillsdale, NJ.
Altmann J (1974). Observational study of behavior: sampling methods. Behavior 49: 227–265.
Altmann SA (1962). A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann NY Acad Sci 102: 338–435.
Bacopoulos NG, Redmond DE, Roth RH (1979). Serotonin and dopamine metabolites in brain regions and cerebrospinal fluid of a primate species: effects of ketamine and fluphenazine. J Neurochem 32: 1215–1218.
Bayart F, Hayashi KT, Faull KF, Barchas JD, Levine S (1990). Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta). Behav Neurosci 104: 98–107.
Berman C (1990). Consistency in maternal behavior within families of free-ranging rhesus monkeys: an extension of the concept of maternal style. Am J Primatol 22: 159–169.
Bowlby J (1982). Attachment and Loss: Attachment, Vol 1. Basic Books, Inc.: New York.
Boyce W, Champoux M, Suomi S, Gunnar M (1995). Salivary cortisol in nursery-reared rhesus monkeys: reactivity to peer interactions and altered circadian activity. Dev Psychobiol 28: 257–267.
Brammer GL, Raleigh MJ, McGuire MT, Rubinstein EH (1987). Comparison of ketamine, physical restraint, halothane and pentobarbital: lack of influence on serotonergic measures in monkeys and rats. Neuropharmacology 26: 1615–1621.
Chalmers N (1984). Social play in monkeys: theories and data. In: Smith PK (ed). Play in Animals and Humans. Blackwell: Oxford. pp 119–141.
Champoux M, Suomi SJ, Schneider ML (1994). Temperament differences between captive Indian and Chinese-Indian hybrid rhesus macaque neonates. Lab Anim Sci 44: 351–357.
Clarke AS, Hedeker DR, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW (1996). Rearing experience and biogenic amine activity in infant rhesus monkeys. Biol Psychiatry 40: 338–352.
Fairbanks LA (1989). Early experience and cross-generational continuity of mother–infant contact in vervet monkeys. Dev Psychobiol 22: 669–681.
Fairbanks LA (1996). Individual differences in maternal style: causes and consequences for mothers and offspring. Adv Study Behav 23: 579–611.
Fairbanks LA, McGuire MT (1988). Long-term effects of early mothering behavior on responsiveness to the environment in vervet monkeys. Dev Psychobiol 21: 711–724.
Fairbanks LA, Melega WP, McGuire MT (1998). CSF 5-HIAA is associated with individual differences in maternal protectiveness in vevet monkeys. Am J Primatol 45: 179–180.
Gerald M, Higley S, Lussier ID, Westergaard GC, Suomi SJ, Highley JD (2002). Variation in reproductive outcomes for captive male rhesus macaques (Macaca mulatta) differing in CSF 5-hydroxyindoleacetic acid concentrations. Brain Behav Ecol 60: 117–124.
Higley JD, Bennett AJ (1999). Central nervous system serotonin and personality as variables contributing to excessive alcohol consumption in non-human primates. Alcohol Alcoholism 34: 402–418.
Higley JD, King ST, Hasert MF, Champoux M, Suomi SJ, Linnoila M (1996a). Stability of interindividual differences in serotonin function and its relationship to aggressive wounding and competent social behavior in rhesus macaque females. Neuropsychopharmacology 14: 67–76.
Higley JD, Linnoila M (1997). Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Ann NY Acad Sci 836: 39–56.
Higley JD, Linnoila M, Suomi SJ (1994). Ethological contributions: experiential and genetic contributions to the expression and inhibition of aggression in primates. In: Hersen M, Ammerman RT, Sisson L (eds). Handbook of Aggressive and Destructive Behavior in Psychiatric Patients. Plenum Press: New York. pp 17–32.
Higley JD, Mehlman P, Taub D, Higley SB, Vickers JH, Suomi SJ et al (1992). Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch Gen Psychiatry 49: 436–441.
Higley JD, Suomi SJ (1996). Effect of reactivity and social competence on individual responses to severe stress in children: investigations using nonhuman primates. In: Pfeffer CR (ed). Intense Stress and Mental Disturbance in Children. American Psychiatric Press, Inc.: Washington, DC. pp 1–69.
Higley JD, Suomi SJ, Linnoila M (1996b). A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcoholism: Clin Exp Res 20: 643–650.
Higley JD, Suomi SJ, Linnoila M (1996c). A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcoholism: Clin Exp Res 20: 629–642.
Higley JD, Thompson WT, Champoux M, Goldman D, Hasert MF, Kraemer GW et al (1993). Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta). Arch Gen Psychiatry 50: 615–623.
Hinde RA, Simpson MJA (1967). Qualities of mother–infant relationships in monkeys. CIBA Found Symp 33: 39–68.
Hinde RA, Spencer-Booth Y (1971). Toward understanding individual differences in rhesus mother–infant interaction. Anim Behav 19: 165–173.
Kraemer GW (1997). Psychobiology of early social attachment in rhesus monkeys. Ann NY Acad Sci 807: 401–418.
Kraemer GW, Clarke A (1996). Social attachment, brain function, and aggression. Ann NY Acad Sci 794: 121–135.
Lee PC (1983). Play as a means for developing relationships. In: Hinde RA (ed). Primate Social Relationships: An Integrated Approach, Vol 1. Sinauer Associates Inc.: Sunderland, MA. pp 82–891.
Lilly AA, Mehlman PT, Higley JD (1999). Trait-like immunological and hematological measures in female rhesus across varied environmental conditions. Am J Primatol 48: 197–223.
Lindell SG, Higley J, Shannon C, Linnoila M (1997). Low levels of CSF 5-HIAA in female rhesus macaques predict mother–infant interaction patterns and mother's CSF 5-HIAA correlates with infant's CSF 5-HIAA. Am J Primatol 42: 129.
Lubach G, Coe CL, Ershler W (1995). Effects of early rearing enivorment on immue responses of infant rhesus monkeys. Brain Behav Immun 9: 31–46.
Maestripieri D (1998). Parenting styles of abusive mothers in group-living rhesus macaques. Anim Behav 55: 1–11.
Maestripieri D, Carroll K (1998). Risk factors for infant abuse and neglect in group-living rhesus monkeys. Psychol Sci 9: 143–145.
Maestripieri D, Megna N, Lindell S, Ayala A, Gold P, Higley J Neurobiological correlates of infant abuse in nonhuman primates and their relation to social and maternal behavior. (unpublished).
Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, Linnoila M (1997). CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiatry Res 72: 89–102.
Meyer JS, Novak MA, Bowman RE, Harlow HF (1975). Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Dev Psychobiol 8: 425–435.
Sanchez M, Hearn E, Do D, Rilling J, Herndon J (1998). Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res 812: 38–49.
Scheinin M, Chang WH, Jimerson DC, Linnoila M (1983). Measurement of 3-methoxy-4-hydroxyphenylglycol in human plasma with high-performance liquid chromatography using electrochemical detection. Anal Biochem 132: 165–170.
Seay B, Harlow HF (1965). Maternal separation in the rhesus monkey. J Nervous Mental Dis 140: 434–444.
Siegel SJ, Ginsberg SD, Hof PR, Foote SL, Young WG, Kraemer GW et al (1993). Effects of social deprivation in prepubescent rhesus monkeys: immunohistochemical analysis of the neurofilament protein triplet in the hippocampal formation. Brain Res 619: 299–305.
Simpson M, Howe S (1997). Group and matriline differences in the behavior of rhesus monkey infants. Anim Behav 34: 444–459.
Smith PK (1982). Does play matter? Functional and evolutionary aspects of animal and human play. Behav Brain Sci 5: 139–184.
Suomi SJ (1973). Surrogate rehabilitation of monkeys reared in total social isolation. Child Psychol Psychiatry 14: 71–77.
Suomi SJ, Collins ML, Harlow HF, Ruppenthal GC (1976). Effects of maternal and peer separations on young monkeys. J Child Psychol Psychiatry 17: 101–112.
Suomi SJ, Kraemer GW, Baysinger CM, DeLizio RD (1981). Inherited and experiential factors associated with individual differences in anxious behavior displayed by rhesus monkeys. In: Klein DF, Rabkin J (eds). Anxiety: New Research and Changing Concepts. Raven Press: New York. pp 179–199.
Suomi SJ, Ripp C (1983). A history of mother-less mother monkey mothering at the University of Wisconsin Primate Laboratory. In: Reite M, Caine N (eds). Child Abuse: The Nonhuman Primate Data. Alan R Liss: New York. pp 50–78.
Suomi SJ, Seaman SF, Lewis JK, DeLizio RD, McKinney WT (1978). Effects of imipramine treatment of separation-induced social disorders in rhesus monkeys. Arch Gen Psychiatry 35: 321–325.
Symons D (1978). Play and Aggression: A Study of Rhesus Monkeys. Columbia University Press: New York.
Taub DM, Mehlman PT (1989). Development of the Morgan Island Rhesus Monkey colony. Puerto Rico Health Sci J 8: 159–169.
van Hooff JARAM (1967). The facial displays of the catarrhine monkeys and apes. In: Morris D (ed). Primate Ethology. Aldine Publishing Company: Chicago. pp 7–68.
Westergaard GC, Cleveland A, Trenkle MK, Lussier ID, Higley JD (2003a). CSF 5-HIAA concentration as an early screening tool for predicting significant life history outcomes in female specific-pathogen-free (SPF) rhesus macaques (Macaca mulatta) maintained in captive breeding groups. J Med Primatol 32: 95–104.
Westergaard GC, Mehlman PT, Shoaf SE, Suomi SJ, Higley JD (1999). CSF 5-HIAA and aggression in female primates: species and interindividual differences. Psychopharmacology 146: 440–446.
Westergaard GC, Suomi SJ, Chavanne T, Houser L, Hurley A, Cleveland A et al (2003b). Physiological correlates of aggression and impulsivity in free-ranging female primates. Neuropsychopharmacology 28: 1045–1055.
Acknowledgements
This research was conducted with support from NIH Grants 5U42RR05083 and R24 RR09983. The authors wish to thank Alecia Lilly, who supervised and assisted in the day-to-day behavior and biochemical data collection under an NIAAA Intramural Fellowship, and Patrick Mehlman who oversaw LABS support during the data collection phase of the project. In addition, our appreciation is expressed to Stephen Lindell, Courtney Shannon, Anne Hurley, Caroline Brewer, Keri Holmes, and to the LABS Animal Care staff who assisted in the biochemical sampling. The LABS of Virginia, Inc. Institutional Animal Care and Use Committee approved a research protocol for this study in accordance with and as required by the Animal Welfare Act.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cleveland, A., Westergaard, G., Trenkle, M. et al. Physiological Predictors of Reproductive Outcome and Mother–Infant Behaviors in Captive Rhesus Macaque Females (Macaca mulatta). Neuropsychopharmacol 29, 901–910 (2004). https://doi.org/10.1038/sj.npp.1300361
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1300361