Abstract
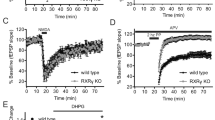
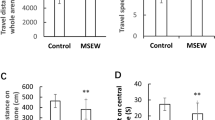
Rearing rats in impoverished (IC) and enriched (EC) environmental conditions alters synaptic plasticity and cognitive processes. Metabotropic glutamate receptors (mGluRs) are known to play a key role in synaptic and behavioral plasticity. In the present study, the effect of rearing conditions on the expression of mGluR proteins in the prefrontal cortex (PFC) was assessed by immunoblotting. A significant difference in the content of prefrontal mGluR1 and mGluR5 (ie group I) and mGluR2/3 (ie group II) was observed between IC and EC rats. To functionally characterize this difference, in vivo microdialysis was used to verify differences in mGluR regulation of extracellular glutamate in the PFC. The results indicate that the capacity of group I and II mGluRs to elevate extracellular glutamate levels was significantly blunted in the PFC of IC rats compared to either EC subjects, or rats reared in normal environmental conditions (ie NIH standards). Group II mGluR receptors regulate performance in a forced T-maze spatial memory task that involves the PFC, and IC rats demonstrated deficits in this task relative to EC rats. These data suggest that reduced mGluR transmission in the PFC produced by impoverished, relative to enriched, rearing environments may contribute to cognitive deficits.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anwyl R (1999). Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29: 83–120.
Aultman JM, Moghaddam B (2001). Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 153: 353–364.
Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW (2002). The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22: 9134–9141.
Bardo MT, Klebaur JE, Valone JM, Deaton C (2002). Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 155: 278–284.
Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL et al (1997). Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386: 284–288.
Bremner JD (1999). Does stress damage the brain? Biol Psychiatry 45: 797–805.
Cartmell J, Schoepp DD (2000). Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75: 889–907.
Chen J, Backus KH, Deitmer J (1997). Intracellular calcium transients and potassium current oscillations evoked by glutamate in cultured rat astrocytes. J Neurosci 17: 7278–7287.
Cochilla AJ, Alford S (1998). Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron 20: 1007–1016.
Escorihuela RM, Tobena A, Fernandez-Teruel A (1995). Environmental enrichment and postnatal handling prevent spatial learning deficits in aged hypoemotional (Roman high-avoidance) and hyperemotional (Roman low-avoidance) rats. Learn Mem 2: 40–48.
Fagni L, Chavis P, Ango F, Bockaert J (2000). Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci 23: 80–88.
Gereau IV RW, Conn PJ (1995). Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci 15: 6879–6889.
Goldman-Rakic PS (1996). Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA 93: 13473–13480.
Goldstein RZ, Volkow ND (2002). Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159: 1642–1652.
Gregory ML, Stech NE, Owens RW, Kalivas PW (2003). Prefrontal group II metabotropic glutamate receptor activation decreases performance on a working memory task. Ann NY Acad Sci 1003: 405.
Hall FS (1998). Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol 12: 129–162.
Heim C, Nemeroff CB (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49: 1023–1039.
Juraska JM, Henderson C, Muller J (1984). Differential rearing experience, gender, and radial maze performance. Dev Psychobiol 17: 209–215.
Kolb B, Whishaw IQ (1998). Brain plasticity and behavior. Annu Rev Psychol 49: 43–64.
Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T et al (2000). Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407: 971–977.
Manzoni O, Michel JM, Bockaert J (1997). Metabotropic glutamate receptors in the rat nucleus accumbens. Eur J Neurosci 9: 1514–1523.
Martinez-Cue C, Baamonde C, Lumbreras M, Paz J, Davisson MT, Schmidt C et al (2002). Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. Behav Brain Res 134: 185–200.
Moghaddam B (2002). Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry 51: 775–787.
Moghaddam B, Adams B, Verma A, Daly D (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17: 2921–2927.
Moghaddam B, Adams BW (1998). Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 28: 1349–1352.
Moroni F, Cozzi A, Lombardi G, Sourtcheva S, Leonardi P, Carfi M et al (1998). Presynaptic mGlu1 type receptors potentiate transmitter output in the rat cortex. Eur J Pharmacol 347: 189–195.
Paxinos G, Watson C (1998). The Rat Brain in Stereotaxic Coordinates 4th edn. Academic Press: New York.
Renner MJ, Rosenzweig MR (1987). Enriched and Impoverished Environments: Effects of Brain and Behavior. Springer-Verlag: New York.
Robinson TE, Whishaw IQ (1988). Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res 450: 209–224.
Romanides AJ, Duffy P, Kalivas PW (1999). Glutamatergic and dopaminergic afferents to the prefrontal cortex regulate spatial working memory in rats. Neuroscience 92: 97–106.
Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW (1995). Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 355: 455–469.
Sato T, Shimada Y, Nagasawa N, Nakanishi S, Jingami H (2003). Amino acid mutagenesis of the ligand binding site and the dimer interface of the metabotropic glutamate receptor 1. Identificaiton of crucial residues for setting the activated state. J Biol Chem. 278: 4314–4321.
Schoepp DD, Jane DE, Monn JA (1999). Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 38: 1431–1476.
Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW (2001). Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci 21: 9043–9052.
Van Praag H, Kempermann G, Gage FH (2000). Neural consequences of environmental enrichment. Nat Rev Neurosci 1: 191–198.
Winterfeld KT, Teuchert-Noodt G, Dawirs RR (1998). Social environment alters both ontogeny of dopamine innervation of the medial prefrontal cortex and maturation of working memory in gerbils (Meriones unguiculatus). J Neurosci Res 52: 201–209.
Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD (2001). 3H] LY341495 binding to group II metabotropic glutamate receptors in rat brain. J Pharmacol Exp Ther 298: 453–460.
Xi Z, Ramamoorthy S, Baker DB, Shen H, Samuvel DJ, Kalivas PW (2002a). Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther 303: 608–615.
Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW (2002b). Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300: 162–171.
Acknowledgements
This work was supported in part by the National Institute of Health Grants MH-40817, DA-07288, and MH-070194.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melendez, R., Lee Gregory, M., Bardo, M. et al. Impoverished Rearing Environment Alters Metabotropic Glutamate Receptor Expression and Function in the Prefrontal Cortex. Neuropsychopharmacol 29, 1980–1987 (2004). https://doi.org/10.1038/sj.npp.1300507
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1300507
Keywords
This article is cited by
-
Early social isolation stress increases addiction vulnerability to heroin and alters c-Fos expression in the mesocorticolimbic system
Psychopharmacology (2022)
-
Promoting neuroplasticity and neuropsychological functioning in frailty through an app-based sensorimotor training: study protocol for a randomized trial
BMC Geriatrics (2021)
-
Early impoverished environment delays the maturation of cerebral cortex
Scientific Reports (2018)
-
Characterization of Behavioral, Signaling and Cytokine Alterations in a Rat Neurodevelopmental Model for Schizophrenia, and Their Reversal by the 5-HT6 Receptor Antagonist SB-399885
Molecular Neurobiology (2018)
-
Effects of the Positive Allosteric Modulator of Metabotropic Glutamate Receptor 5, VU-29, on Impairment of Novel Object Recognition Induced by Acute Ethanol and Ethanol Withdrawal in Rats
Neurotoxicity Research (2018)