Abstract
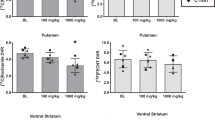
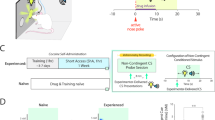
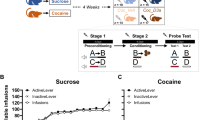
Cocaine acts as a reinforcer through its pharmacological effects on brain monoaminergic systems, which, through repeated pairings with environmental stimuli, lead to the development of conditioned effects of the drug. Both the pharmacological and conditioned aspects of cocaine are implicated in several facets of acquisition and maintenance of addiction, including drug craving. Here, we compare the effects of contingent (response dependent) and noncontingent (response independent) cocaine on rapid dopaminergic signaling in the core of the nucleus accumbens. Dopamine was monitored using fast-scan cyclic voltammetry. Noncontingent cocaine administered to both naïve and animals with a history of self-administration resulted in a profound increase in the frequency of transient dopamine release events that are not time-locked to any specific environmental stimuli. Pharmacological effects were detectable approximately 40 s after cocaine administration. In contrast, when animals where allowed to self-administer cocaine on an FR-1 schedule, dopamine transients (69±12 nM) were consistently observed time-locked to each reinforced response (peaking approximately 1.5 s after response completion). Importantly, no pharmacological effect of cocaine was observed within the 10 s following noncontingent cocaine administration, indicating that dopamine signals time-locked to the reinforced response are a result of the pairing of the operant behavior, the drug-associated cues, and cocaine. These data demonstrate that this pharmacological action of cocaine occurs for an extended period following either contingent or noncontingent administration, but is distinct from those dopamine transients that are time-locked to each lever-press in self-administering animals.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003). Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464: 220–237.
Bradberry CW (2000). Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci 20: 7109–7115.
Bunney EB, Appel SB, Brodie MS (2000). Cocaine potentiates ethanol-induced excitation of dopaminergic reward neurons in the ventral tegmental area. J Pharmacol Exp Ther 293: 383–389.
Caine SB, Koob GF (1994). Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270: 209–218.
Carelli RM (2004). Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology 47(Suppl 1): 180–189.
Carelli RM, Deadwyler SA (1996). Dose-dependent transitions in nucleus accumbens cell firing and behavioral responding during cocaine self-administration sessions in rats. J Pharmacol Exp Ther 277: 385–393.
Carelli RM, Ijames SG, Crumling AJ (2000). Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus ‘natural’ (water and food) reward. J Neurosci 20: 4255–4266.
Chang JY, Sawyer SF, Lee RS, Woodward DJ (1994). Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci 14: 1224–1244.
Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM (2004). Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci 24: 4393–4400.
Ciccocioppo R, Martin-Fardon R, Weiss F (2004). Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat Neurosci 7: 495–496.
Dackis CA, O'Brien CP (2001). Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat 21: 111–117.
Di Chiara G (2002). Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137: 75–114.
Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C et al (2004). Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47(Suppl 1): 227–241.
Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278.
Einhorn LC, Johansen PA, White FJ (1988). Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci 8: 100–112.
Everitt BJ, Robbins TW (2000). Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 153: 17–30.
Floresco SB, West AR, Ash B, Moore H, Grace AA (2003). Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6: 968–973.
Garris PA, Christensen JR, Rebec GV, Wightman RM (1997). Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem 68: 152–161.
Garris PA, Collins LB, Jones SR, Wightman RM (1993). Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J Neurochem 61: 637–647.
Gawin FH (1991). Cocaine addiction: psychology and neurophysiology. Science 251: 1580–1586.
Gessa GL, Melis M, Muntoni AL, Diana M (1998). Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 341: 39–44.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996). Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379: 606–612.
Gratton A, Wise RA (1994). Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci 14: 4130–4146.
Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM (2003). Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 128: 1413–1419.
Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R (2002). Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 114: 475–492.
Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ (2000). Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 20: 7489–7495.
Johnson LR, Aylward RL, Hussain Z, Totterdell S (1994). Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience 61: 851–865.
Jones SR, Garris PA, Wightman RM (1995). Different effects of cocaine and nomifensine on dopamine uptake in the Caudate-Putamen and nucleus accumbens. J Pharmacol Exp Ther 274: 396–403.
Kitai ST, Shepard PD, Callaway JC, Scroggs R (1999). Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol 9: 690–697.
Kiyatkin EA, Stein EA (1995). Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience 64: 599–617.
Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A et al (2004). Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27: 739–749.
Kuhr WG, Bigelow JC, Wightman RM (1986). In vivo comparison of the regulation of releasable dopamine in the caudate nucleus and the nucleus accumbens of the rat brain. J Neurosci 6: 974–982.
Nicola SM, Surmeier J, Malenka RC (2000). Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185–215.
O'Brien CP, Childress AR, McLellan AT, Ehrman R (1992). Classical conditioning in drug-dependent humans. Ann NY Acad Sci 654: 400–415.
O'Donnell P, Greene J, Pabello N, Lewis BL, Grace AA (1999). Modulation of cell firing in the nucleus accumbens. Ann NY Acad Sci 877: 157–175.
Paladini CA, Mitchell JM, Williams JT, Mark GP (2004). Cocaine self-administration selectively decreases noradrenergic regulation of metabotropic glutamate receptor-mediated inhibition in dopamine neurons. J Neurosci 24: 5209–5215.
Pan HT, Menacherry S, Justice Jr JB (1991). Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem 56: 1299–1306.
Parkinson JA, Cardinal RN, Everitt BJ (2000). Limbic cortical–ventral striatal systems underlying appetitive conditioning. Prog Brain Res 126: 263–285.
Paxinos G, Watson C (1997). The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego.
Pennartz CM, Groenewegen HJ, Lopes da Silva FH (1994). The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol 42: 719–761.
Pettit HO, Justice Jr JB (1989). Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav 34: 899–904.
Pettit HO, Justice Jr JB (1991). Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res 18: 94–102.
Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM (2003a). Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med 79: 443–464.
Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003b). Subsecond dopamine release promotes cocaine seeking. Nature 422: 614–618.
Phillips PE, Wightman RM (2003). Critical guidelines for validation of the selectivity of in-vivo chemical microsensors. Trends Anal Chem 22: 509–514.
Rebec GV, Christensen JR, Guerra C, Bardo MT (1997). Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res 776: 61–67.
Richfield EK, Penney JB, Young AB (1989). Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30: 767–777.
Robinson DL, Heien ML, Wightman RM (2002). Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci 22: 10477–10486.
Robinson DL, Wightman RM (2004). Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. J Neurochem 90: 894–903.
Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM (2004). Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24: 1265–1271.
Salamone JD, Correa M (2002). Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res 137: 3–25.
Schultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27.
See RE, Fuchs RA, Ledford CC, McLaughlin J (2003). Drug addiction, relapse, and the amygdala. Ann NY Acad Sci 985: 294–307.
Sesack SR, Pickel VM (1990). In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res 527: 266–279.
Shi WX, Pun CL, Zhou Y (2004). Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology 29: 2160–2167.
Stewart J (1992). Neurobiology of conditioning to drugs of abuse. Ann NY Acad Sci 654: 335–346.
Totterdell S, Smith AD (1989). Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat 2: 285–298.
Uzwiak AJ, Guyette FX, West MO, Peoples LL (1997). Neurons in accumbens subterritories of the rat: phasic firing time-locked within seconds of intravenous cocaine self-infusion. Brain Res 767: 363–369.
Venton BJ, Michael DJ, Wightman RM (2003b). Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. J Neurochem 84: 373–381.
Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM (2003a). Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem 87: 1284–1295.
Volkow ND, Fowler JS, Wang GJ (2003). The addicted human brain: insights from imaging studies. J Clin Invest 111: 1444–1451.
Waelti P, Dickinson A, Schultz W (2001). Dopamine responses comply with basic assumptions of formal learning theory. Nature 412: 43–48.
Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben Shahar O (2000). Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA 97: 4321–4326.
Wightman RM, Robinson DL (2002). Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem 82: 721–735.
Wise RA (1982). Common neural basis for brain stimulation reward, drug reward, and food reward. In BG Hoebel, D Novin (eds). The Neural Basis of Feeding and Reward. Haer Institute: Brunswick, ME. pp 445–454.
Wise RA (2004). Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–494.
Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice Jr JB (1995). Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 120: 10–20.
Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA (2001). Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci 21: 6338–6347.
Acknowledgements
The authors thank MLAV Heien, SE Brooks, JH Wondolowski, and the UNC Electronics Facility. This work was supported by grants from the National Institute on Drug Abuse; DA10900 (RMW), DA17318 (RMC & RMW), and DA015923 (GDS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stuber, G., Roitman, M., Phillips, P. et al. Rapid Dopamine Signaling in the Nucleus Accumbens during Contingent and Noncontingent Cocaine Administration. Neuropsychopharmacol 30, 853–863 (2005). https://doi.org/10.1038/sj.npp.1300619
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1300619
Keywords
This article is cited by
-
Measuring neuron-regulated immune cell physiology via the alpha-2 adrenergic receptor in an ex vivo murine spleen model
Cellular and Molecular Life Sciences (2023)
-
Rate of onset of dopamine transporter inhibitors assessed with intracranial self-stimulation and in vivo dopamine photometry in rats
Psychopharmacology (2023)
-
Cocaine induces paradigm-specific changes to the transcriptome within the ventral tegmental area
Neuropsychopharmacology (2021)
-
Regulation of dopamine-dependent transcription and cocaine action by Gadd45b
Neuropsychopharmacology (2021)
-
Accelerated development of cocaine-associated dopamine transients and cocaine use vulnerability following traumatic stress
Neuropsychopharmacology (2020)