Abstract
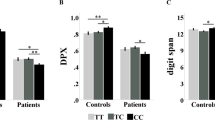
A recently discovered gene complex, G72/G30 (hereafter G72, but now termed DAOA), was found to be associated with schizophrenia and with bipolar disorder, possibly because of an indirect effect on NMDA neurotransmission. In principle, if G72 increases risk for psychosis by this mechanism, it might impact with greater penetrance those cortically based cognitive and neurophysiological functions associated with NMDA signaling. We performed two independent family-based association studies (one sample contained more than 200 families and the other more than 65) of multiple SNPs in the G72 region and of multiple SNPs in the gene for D-amino acid oxidase (DAAO), which may be modulated by G72. We examined the relationship between select cognitive measures in attention, working memory, and episodic memory and a restricted set of G72 SNPs in over 600 normal controls, schizophrenic patients, and their nonpsychotic siblings using mixed model ANOVAs. We also determined genotype effects on neurophysiology measures in normal controls using the fMRI BOLD response obtained during activation procedures involving either episodic memory or working memory. There were no significant single G72 SNP associations and clinical diagnosis in either sample, though one approached significance (p=0.06). Diagnosis by genotype interaction effects for G72 SNP 10 were significant for cognitive variables assessing working memory and attention (p=0.05), and at the trend level for episodic memory, such that in the schizophrenia group an exaggerated allele load effect in the predicted directions was observed. In the fMRI paradigms, a strong effect of G72 SNP 10 genotype was observed on BOLD activation in the hippocampus during the episodic memory paradigm. Tests of association with DAAO were consistently nonsignificant. We present evidence that SNP variations in the G72 gene region increase risk of cognitive impairment in schizophrenia. SNP variations were not strongly associated with clinical diagnosis in family-based analyses.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Addington AM, Gornick M, Sporn AL, Gogtay N, Greeenstein D, Lenane M et al (2004). Polymorphisms in the 13q33.2 gene G72/G30 are associated with childhood-onset schizophrenia and psychosis not otherwise specified. Biol Psychiatry 55: 976–980.
Brett M (2004). www.mrc-cbu.cam.ac.uk/Imaging/mnispace.htmlCambridge, England.
Burggren AC, Small GW, Sabb FW, Bookheimer SY (2002). Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am J Geriatr Psychiatry 10: 44–51.
Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R et al (2000). Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 10: 1078–1092.
Chen YS, Akula N, Detera-Wadleigh SD (2004). Findings in an independent sample support an association between bipolar affective disorder and the G72/G30 locus on chromosome 13q33. Molec Psychiatry 9: 87–92.
Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H et al (2002). Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99: 13675–13680.
Clayton D (2001). TRANSMIT v. 2.5. Cambridge, England.
Cloninger CR, Kaufmann CA, Faraone SV, Malaspina D, Svrakic DM, Harkavy-Friedman J et al (1998). Genome-wide search for schizophrenia susceptibility loci: the NIMH Genetics Initiative and Millennium Consortium. Am J Med Genet 81: 275–281.
Cox NJ (2002). Calpain 10 and genetics of type 2 diabetes. Curr Diab Rep 2: 186–190.
Deakin JF, Slater P, Simpson MD, Gilchrist AC, Skan WJ, Royston MC et al (1989). Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. J Neurochem 52: 1781–1786.
Doniger GM, Silipo G, Rabinowicz EF, Snodgrass JG, Javitt DC (2001). Impaired sensory processing as a basis for object-recognition deficits in schizophrenia. Am J Psychiatry 158: 1818–1826.
Egan M, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Bigelow L et al (2001a). Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry 50: 98–107.
Egan MF, Goldberg TE, Kojima M, Callicott J, Kolachan BS, Bertolino A et al (2003). The BDNF val66met polymorphism affects activity dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269.
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE et al (2001b). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98: 6917–6922.
Elvevaag B, Weinberger DR, Suter J, Goldberg TE (2000). The Continuous Performance Test and schizophrenia: a test of stimulus response compatibility, working memory, or response readiness? Am J Psychiatry 157: 772–780.
Gabrieli JD, Preston AR (2003). Visualizing genetic influences on human brain function. Cell 112: 144–145.
Gainetdinov RR, Mohn AR, Caron MG (2001). Genetic animal models: focus on schizophrenia. Trends Neurosci 24: 527–533.
Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D et al (1996). Quantitative MRI of the temporal lobe, amygdale, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol 366: 223–230.
Goff DC, Coyle JT (2001). The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158: 1367–1377.
Goldberg TE, Egan MF, Gsceidle T, Weickert T, Coppola RC, Kolachana BS et al (2003). Executive subprocesses in working memory: Relationships with COMT Val/Met genotype and genetic risk for schizophrenia. Arch Gen Psychiatry 60: 889–896.
Goldberg TE, Green MF (2002). Neurocognitive functioning in patients with schizophrenia: An overview. In Davis KL (ed). Psychopharmacology: The Fifth Generation of Progress. Raven Press: New York. pp 657–669.
Goldberg TE, Ragland DR, Gold J, Bigelow LB, Torrey EF, Weinberger DR (1990). Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 47: 1066–1072.
Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF et al (2003). Brain derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 23: 6690–6694.
Hariri AR, Weinberger DR (2003). Imaging genomics. Br Med Bull 65: 237–248.
Harrison PJ, Owen MJ (2003). Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet 361: 417–419.
Hattori E, Chunyu L, Badner JA, Bonner TI, Christian SL, Maheshwari M et al (2003). Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am J Hum Genet 72: 1131–1140.
Jentsch JD, Roth RH (1999). The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20: 201–225.
Kaufman AS (1990). Assessing Adolescent and Adult Intelligence. Allyn Bacon: New York, 753 pp.
Krystal JH, Anand A, Moghaddam B (2002). Effects of NMDA receptor antagonists: implications for the pathology of schizophrenia. Arch Gen Psychiatry 59: 663–664.
Krystal JH, Bennett A, Abi-Saab D, Belger A, Karper LP, D'Souza DC et al (2000). Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry 15: 137–143.
Krystal JH, Karper LP, Bennett A, D'Souza DC, Abi-Dargham A, Morrissey K et al (1998). Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Biol Psychiatry 135: 213–229.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214.
Lang PJ, Bradley MM, Cuthbert BN (1997). International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention Gainesville, FL.
Lin Y, Fossom LH, Skolnick P, Long JB (1998). Sustained exposure to a glycine recpetor partial agonist differentially alters NMDA receptor agonist and antagonist potencies of cultured spinal cord neurons. Eur J Pharmacol 356: 255–260.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996). SAS System for Mixed Models. Carey, NC.
Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN (2003). Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 33: 177–182.
Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D et al (1996). NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 14: 301–307.
Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN et al (2002). Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol 51: 156–164.
Meador-Woodruff JH, Healy DJ (2000). Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev 31: 288–294.
Pittenger C, Kandel ER (2003). In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos Trans R Soc Lond B Biol Sci 358: 757–763.
Purcell S (2003). Statgen Genetic Power Calculator Module software. Cambridge, England.
Schroeder CE, Lindsley RW, Specht C, Marcovici A, Smiley JF, Javitt DC (2001). Somatosensory input to auditory association cortex in the macaque monkey. J Neurophysiol 85: 1322–1327.
Schumacher J, Jamra R, Freudenberg J (2004). Examination of G72 and d-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Molec Psychiatry 9: 203–207.
Talaraich J, Tournoux P (1988). Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers: New York, 122 pp.
Tsai G, Coyle JT (2002). Glutamatergic mechanisms in schizophrenia. Ann Rev Pharmacol Toxicol 42: 165–179.
Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE et al (1995). Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry 52: 829–836.
Weickert T, Egan MF, Weinberger DR, Goldberg TE (2000). Preserved and compromised intellect in schizophrenia: Neurocognitive implications. Arch Gen Psychiatry 57: 907–913.
Wellcome Department of Cognitive Neurology (2000). SPM99. Cambridge, England.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldberg, T., Straub, R., Callicott, J. et al. The G72/G30 Gene Complex and Cognitive Abnormalities in Schizophrenia. Neuropsychopharmacol 31, 2022–2032 (2006). https://doi.org/10.1038/sj.npp.1301049
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301049
Keywords
This article is cited by
-
Effect of DAOA genetic variation on white matter alteration in corpus callosum in patients with first-episode schizophrenia
Brain Imaging and Behavior (2021)
-
The role of trauma experiences, personality traits, and genotype in maintaining posttraumatic stress disorder symptoms among child survivors of the Wenchuan earthquake
BMC Psychiatry (2020)
-
G72 primate-specific gene: a still enigmatic element in psychiatric disorders
Cellular and Molecular Life Sciences (2016)
-
Identification of the Mitochondrial MSRB2 as a Binding Partner of LG72
Cellular and Molecular Neurobiology (2014)
-
d-amino acid oxidase activator gene (DAOA) variation affects cerebrospinal fluid homovanillic acid concentrations in healthy Caucasians
European Archives of Psychiatry and Clinical Neuroscience (2012)