Abstract
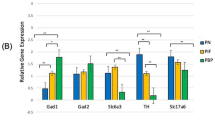
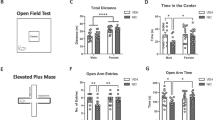
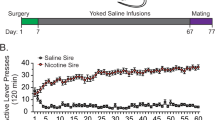
Prenatal nicotine exposure (PNE) has been associated with increased prevalence of attention deficit hyperactivity disorder (ADHD), major depressive disorder (MDD) and substance abuse in exposed children and adolescents. Whether these syndromes are caused by nicotine exposure, or genetic and psychosocial adversities associated with maternal smoking is not completely clear. Animal models suggest a direct impact of PNE. However, the fact that nicotine is forcefully administrated in these paradigms raises some questions about the specificity of these findings. Pregnant C57BI/6J mice were allowed to choose drinking saccharin/nicotine solutions or pure water. Controls could choose saccharin solutions or pure water. Offspring were tested in spontaneous locomotion, fear-associated learning (trace conditioning), addictive (conditioned place preference), and depression-like (learned helplessness) behaviors. There was no significant difference in weight or pup number between the prenatal treatment groups. A significant effect of PNE was observed on spontaneous locomotion, preference for a cocaine-associated place, and latency to escape in the learned helplessness paradigm. Surprisingly, PNE mice exhibited an increased learning of trace-conditioned fear-associated cues. The hyperlocomotive behavior reported in animal models of PNE is not likely an artifact of forceful nicotine administration. The increased prevalence of ADHD, MDD and substance abuse observed in PNE children and adolescents is probably caused by direct behavioral teratogenic effects of PNE. The role of PNE as a risk factor of syndromes associated to increased learning of fear-associated cues such as post-traumatic stress disorder (PTSD) warrants further evaluation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adler LA, Kunz M, Chua HC, Rotrosen J, Resnick SG (2004). Attention-deficit/hyperactivity disorder in adult patients with posttraumatic stress disorder (PTSD): Is ADHD a vulnerability factor? J Atten Disord 8: 11–16.
Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G (2004). Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology 29: 869–878.
Allan AM, Engel SR, Savage DD, Galindo R, Cynoweth J (2001). Conditioned place preference for cocaine is attenuated in mice over-expressing the 5-HT3 receptor. Psychopharmacology 158: 18–27.
Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306: 879–881.
Caldarone BJ, George TP, Zachariou V, Picciotto MR (2000). Gender differences in learned helplessness behavior are influenced by genetic background. Pharmacol Biochem Behav 66: 811–817.
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H et al (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301: 386–389.
Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA (2001). Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J Dev Behav Pediatr 22: 217–225.
Ernst M, Moolchan ET, Robinson ML (2001). Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry 40: 630–641.
Eskenazi B, Prehn AW, Christianson RE (1995). Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight. Am J Public Health 85: 395–398.
Fendt M, Fanselow MS, Koch M (2005). Lesions of the dorsal hippocampus block trace fear conditioned potentiation of startle. Behav Neurosci 119: 834–838.
Fergusson DM, Woodward LJ, Horwood LJ (1998). Maternal smoking during pregnancy and psychiatric adjustments in late adolescence. Arch Gen Psychiatry 55: 721–727.
Fried PA, Watkinson B, Gray R (2003). Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 25: 427–436.
Genedani S, Bernardi M, Bertolini A (1983). Sex-linked differences in avoidance learning in the offspring of rats treated with nicotine during pregnancy. Psychopharmacology (Berlin) 80: 93–95.
Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L et al (2002). Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416: 396–400.
Han CJ, O'Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R et al (2003). Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci USA 100: 13087–13092.
Hess EJ, Collins KA, Wilson MC (1996). Mouse model of hyperkinesis implicates SNAP-25 in behavioral regulation. J Neurosci 16: 3104–3111.
King SL, Marks MJ, Grady SR, Caldarone BJ, Koren AO, Mukhin AG et al (2003). Conditional Expression in Corticothalamic Efferents Reveals a Developmental Role for Nicotinic Acetylcholine Receptors in Modulation of Passive Avoidance Behavior. J Neurosci 23: 3837–3843.
Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM (2004). Sex differences in voluntary nicotine consumption by adolescent mice: a dose–response experiment. Pharmacol Biochem Behav 78: 13–25.
Kofman O (2002). The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev 26: 457–470.
Lee M, Chen K, Shih JC, Hiroi N (2004). MAO-B knockout mice exhibit deficient habituation of locomotor activity but normal nicotine intake. Genes Brain Behav 3: 216–227.
Li XC, Karadsheh MS, Jenkins PM, Stitzel JA (2005). Genetic correlation between the free-choice oral consumption of nicotine and alcohol in C57BL/6JxC3H/HeJ F2 intercross mice. Behav Brain Res 157: 79–90.
Linnet KM, Wisborg K, Obel C, Secher NJ, Thomsen PH, Agerbo E et al (2005). Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics 116: 462–467.
Martin JA, Kochanek KD, Strobino DM, Guyer B, MacDorman MF (2005). Annual summary of vital statistics. Pediatrics 115: 619–634.
Maughan B, Taylor A, Caspi A, Moffitt TE (2004). Prenatal smoking and early childhood conduct problems: testing genetic and environmental explanations of the association. Arch Gen Psychiatry 61: 836–843.
Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S (2002). Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry 41: 378–385.
Milberger S, Biederman J, Faraone SV, Chen L, Jones J (1996). Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry 153: 1138–1142.
Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE et al (1989). Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther 251: 894–900.
Olsen J (1992). Cigarette smoking in pregnancy and fetal growth Does the type of tobacco play a role? Int J Epidemiol 21: 279–284.
Sibille E, Lewis DA (2006). SERT-ainly involved in depression, but when? Am J Psychiatry 163: 8–11.
Slawecki CJ, Samson HH, Hodge CW (1997). Differential changes in sucrose/ethanol and sucrose maintained responding by independently altering ethanol or sucrose concentration. Alcohol-Clin Exp Res 21: 250–260.
Slotkin TA (1998). Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther 285: 931–945.
Slotkin TA (2004). Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol 198: 132–151.
Slotkin TA, Tate CA, Cousins MM, Seidler FJ (2005). Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: serotonin receptors and cell signaling. Neuropsychopharmacology, 7 December [Epub ahead of print].
Sparks JA, Pauly JR (1999). Effects of continuous oral nicotine administration on brain nicotinic receptors nad responsiveness to nicotine in C57Bl/6 mice. Psychopharmacolgy 141: 145–153.
Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H et al (2003). Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry 160: 1985–1989.
Weissman MM, Warner V, Wickramaratne PJ, Kandel DB (1999). Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry 38: 892–899.
Wu LT, Anthony JC (1999). Tobacco smoking and depressed mood in late childhood and early adolescence. Am J Pub Health 89: 1837–1840.
Xu Z, Seidler FJ, Ali SF, Slikker Jr W, Slotkin TA (2001). Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res 914: 166–178.
Zahalka EA, Seidler FJ, Lappi SE, McCook EC, Yanai J, Slotkin TA (1992). Deficits in development of central cholinergic pathways caused by fetal nicotine exposure: differential effects on choline acetyltransferase activity and [3H] hemicholinium-3 binding. Neurotoxicol Teratol 14: 375–382.
Acknowledgements
We thank Julie Chynoweth for her technical assistance in generating the nicotine exposed offspring for these studies. We also thank Dr John (Jake) McDonald at The Lovelace Respiratory Research Institute in Albuquerque, New Mexico and The New Mexico Center for Environmental Health Sciences (ES P30-012072-05) for analysis of the nicotine plasma levels. This work was supported by Tobacco Settlement Funds through University of New Mexico Research Allocation Committee provided to AMA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paz, R., Barsness, B., Martenson, T. et al. Behavioral Teratogenicity Induced by Nonforced Maternal Nicotine Consumption. Neuropsychopharmacol 32, 693–699 (2007). https://doi.org/10.1038/sj.npp.1301066
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301066
Keywords
This article is cited by
-
Secondhand smoke’s effects on brain development: ADHD and associated behaviors in children
Journal of Umm Al-Qura University for Applied Sciences (2023)
-
Systematic Review and Meta-analysis of the Relationship Between Exposure to Parental Substance Use and Attention-Deficit/Hyperactivity Disorder in Children
Prevention Science (2023)
-
The Intergenerational Transmission of Developmental Nicotine Exposure-Induced Neurodevelopmental Disorder-Like Phenotypes is Modulated by the Chrna5 D397N Polymorphism in Adolescent Mice
Behavior Genetics (2021)
-
Developmental nicotine exposure engenders intergenerational downregulation and aberrant posttranslational modification of cardinal epigenetic factors in the frontal cortices, striata, and hippocampi of adolescent mice
Epigenetics & Chromatin (2020)
-
Transgenerational transmission of behavioral phenotypes produced by exposure of male mice to saccharin and nicotine
Scientific Reports (2020)