Abstract
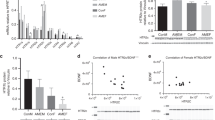
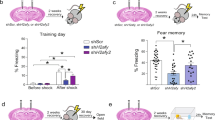
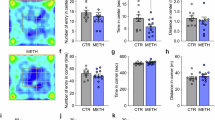
As prenatal methamphetamine (MA) exposure results in long-term hippocampus-dependent cognitive deficits, the increased MA use in women of childbearing age is of great concern. As mice are most commonly used in genetic models, we started to study the potential effects of neonatal MA exposure in female and male mice on brain function 3 months later. As histamine (HA) might mediate some effects of MA in adulthood, we also tested whether in neonates HA might mediate the long-term effects of MA using HA H3 receptor agonists and antagonists. Stimulation of HA H3 receptors by H3 agonists inhibits HA synthesis and release, whereas inhibition of H3 receptors by H3 receptor antagonists increases HA release. MA (5 mg/kg), the H3 receptor antagonist thioperamide (5 mg/kg), and the H3 receptor agonist immepip (5 mg/kg) alone or in the presence of MA (5 mg/kg) were administered once daily from postnatal days 11 to 20 and the mice were tested at 3 months of age. Here we show that in mice exposure to MA early in life causes sex-dependent impairments in object recognition, spatial learning, and memory in the water maze, and pre-pulse inhibition in adulthood. HA mediates these impairments. Increasing HA release mimicked, whereas inhibiting HA release blocked the long-term detrimental MA effects. This model could be used to determine the role of genetic and environmental factors in MA-dependent cognitive impairments and to develop therapeutic strategies to inhibit them.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anglin M, Burke C, Perrochet B, Stamper E, Dawud-Noursi S (2000). History of the methamphetamine problem. J Psychoact Drugs 32: 137–141.
Bayer SA, Altman J, Russo R, Zhang X (1993). Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14: 83–144.
Benice T, Rizk A, Pfankuch T, Kohama S, Raber J (2006). Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 137: 413–423.
Cappon G, Morford L, Vorhees C (1997). Ontogeny of methamphetamine-induced neurotoxicity and associated hyperthermic response. Dev Brain Res 103: 155–162.
Cernerud L, Eriksson M, Johnsson B, Steneroth G, Zetterstrom R (1996). Amphetamine addiction during pregnancy: 14 year follow up of growth and school performance. Acta Pediatr 85: 204–208.
Chang L, Smith L, LoPresti C, Yonekura M, Kuo J, Walot I et al (2004). Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatr Res 132: 95–106.
Dai H, Okuda H, Iwabuchi K, Sakurai E, Chen Z, Kato M et al (2004). Social isolation stress significantly enhanced the disruption of pre-pulse inhibition in mice repeatedly treated with methamphetamine. Ann NY Acad Sci 1025: 257–266.
Dixon S, Bejar R (1989). Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J Pediatr 115: 770–778.
Ennaceur A, Neave N, Aggleton J (1997). Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113: 509–519.
Gomez-Da-Silva J, De Miguel R, Fernandez-Ruiz J, Summavielle T, Tavares MA (2004). Effects of neonatal exposure to methamphetamine: catecholamine levels in brain areas of the developing rat. Ann NY Acad Sci 1025: 602–611.
Hansen R, Struthers J, Gospe S (1993). Visual evoked potentials and visual processing in stimulant drug-exposed infants. Dev Med Child Neurol 35: 798–805.
Ito C (2004). The role of the central histaminergic system on schizophrenia. Drug News Perspect 17: 383–387.
Ito C, Onodera K, Watanabe H, Sato M (1997). Effects of histamine agents on methamphetamine-induced stereotyped behavior and behavioral sensitization in rats. Psychopharmacology 130: 362–367.
Jansen FP, Mochizuki T, Yamamoto Y, Timmerman H, Yamatodani A (1998). In vivo modulation of rat hypothalamic histamine release by the histamine H3 receptor ligands, immepip and clobenpropit. Effects of intrahypothalamic and peripheral application. Eur J Pharamacol 362: 149–155.
Little B, Snell M, Gilstrap III L (1988). Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet Gynecol 75: 541–544.
Marwick C (2000). NIDA seeking data on effect of fetal exposure to methamphetamine. J Am Med Assoc 283: 2225–2226.
Mori T, Narita M, Onodera K, Suzuki T (2002). Modulation of the discriminative stimulus effects of cocaine and methamphetamine by the histaminergic system. Nihon Shinkei Seishin Yakurigaku Zasshi 22: 73–78.
Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark X et al (2000). High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature 408: 860–864.
Morris R (1981). Spatial localization does not require the presence of local cues. Learn Motivation 12: 239–260.
Munzar P, Nosal R, Goldberg SR (1998). Potentiation of the discrimination-stimulus effects of methamphetamines by the histamine H3 receptor antagonist thiopermide in rats. Eur J Pharmacol 363: 93–101.
Munzar P, Tanada G, Justinova Z, Goldberg SR (2004). Histamine H3 receptor antagonist potentiate methamphetamine self-administration and methamphetamine-induced accumbal dopamine release. Neuropsychopharmacology 29: 705–717.
Oro A, Dixon S (1987). Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr 111: 571–578.
Plappert C, Pilz P, Schnitzler H-U (2004). Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behav Brain Res 152: 403–412.
Rice D, Barone JS (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108: 511–533.
Rizk A, Curley J, Robertson J, Raber J (2004). Anxiety and cognition in histamine H3 receptor. Eur J Neurosci 19: 1992–1996.
Save E, Poucet B, Foreman N, Buhot M (1992). Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci 106: 447–456.
Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C (2003). Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Ped 24: 17–23.
Smith R, Dring L (1970). Patterns of Metabolisms of Beta-Phenylisopropylamines in Man and Other Species. Raven Press: New York.
Struthers J, Hansen R (1992). Visual recognition memory in drug-exposed infants. J Dev Behav Perdiatr 13: 108–111.
Vorhees C, Ahrens K, Acuff-Smith K, Schilling M, Fisher J (1994). Methamphetamine exposure during early post-natal development in rats: I. acoustic startle augmentation and spatial learning deficits. Psychopharmacology 114: 392–401.
Vorhees C, Reed T, Schilling M, Acuff-Smith K, Fisher J, Moran M (1996). Neonatal methamphetamine-induced long-term acoustic startle facilitation in rats as a function of prepulse stimulus intensity. Neurotoxicol Teratol 18: 135–139.
Williams M, Moran M, Vorhees C (2004). Behavioral and growth effects induced by low dose methamphetamine during the neonatal period in rats. Int J Dev Neurosci 22: 273–283.
Williams MT, Moran MS, Vorhees CV (2003a). Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology 168: 329–338.
Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW et al (2003b). Developmental D-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse 48: 138–148.
Won L, Bubula N, McCoy H, Heller A (2001). Methamphetamine concentration in fetal and maternal brain following prenatal exposure. Neurotoxicol Teratol 23: 349–354.
Acknowledgements
We thank Dr Gregory Mark for discussion of the methamphetamine dose to use and for reading the manuscript. We thank Timothy Pfankuch, Angela Rizk-Jackson, and Cara Poage for assistance with the water maze experiments. This work was supported by NIH R01 AG20904, EMF AG-NS-0201, and NIDA training grant T32-DA07262-14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acevedo, S., de Esch, I. & Raber, J. Sex- and Histamine-Dependent Long-Term Cognitive Effects of Methamphetamine Exposure. Neuropsychopharmacol 32, 665–672 (2007). https://doi.org/10.1038/sj.npp.1301091
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301091
Keywords
This article is cited by
-
Sex differences in behavior, cognitive, and physiological recovery following methamphetamine administration
Psychopharmacology (2024)
-
Age-Related Differences in NMDA Receptor Subunits of Prenatally Methamphetamine-Exposed Male Rats
Neurochemical Research (2014)
-
An Evaluation of the Evidence that Methamphetamine Abuse Causes Cognitive Decline in Humans
Neuropsychopharmacology (2013)