Abstract
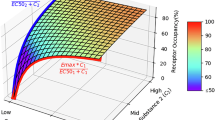
Clozapine and quetiapine have a low incidence of extrapyramidal side effects at clinically effective doses, which appears to be related to their significantly lower occupancy of striatal dopamine D2 receptors (DA D2r) compared to typical antipsychotic drugs (APDs). Animal studies have indicated that clozapine and quetiapine produce selective effects on cortical and limbic regions of the brain and in particular on dopaminergic neurotransmission in these regions. Previous PET and SPECT studies have reported conflicting results regarding whether clozapine produces preferential occupancy of cortical DA D2r. To examine whether clozapine and/or quetiapine produce preferential occupancy of DA D2r in cortex and limbic regions, we studied the occupancy of putamenal, ventral striatal, thalamic, amygdala, substantia nigra, and temporal cortical DA D2r using PET with [18F]fallypride in six schizophrenic subjects receiving clozapine monotherapy and in seven schizophrenic subjects receiving quetiapine monotherapy. Doses were chosen clinically to minimize psychopathology at tolerable levels of side effects such as drowsiness. All had minimal positive symptoms at the time of the study. Regional receptor occupancies were estimated using mean regional DA D2r levels calculated for 10 off-medication schizophrenic subjects. Both clozapine and quetiapine produced lower levels of putamenal DA D2r occupancy than those reported for typical APDs, 47.8 and 33.5%, respectively. Clozapine produced preferential occupancy of temporal cortical vs putamenal DA D2r, 59.8% (p=0.05, corrected for multiple comparisons), and significantly lower levels of occupancy in the substantia nigra, 18.4% (p=0.0015, corrected for multiple comparisons). Quetiapine also produced preferential occupancy of temporal cortical DA D2r, 46.9% (p=0.03, corrected for multiple comparisons), but did not spare occupancy of substantia nigra DA D2r. The therapeutic effects of clozapine and quetiapine appear to be achieved at less than the 65% threshold for occupancy seen with typical APDs, consistent with the involvement of non-DA D2r mechanisms in at least partially mediating the therapeutic effects of these drugs. Preferential occupancy of cortical DA D2r, sparing occupancy of substantia nigra receptors, and non-DA D2r-mediated actions may contribute to the antipsychotic actions of these and other atypical APDs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Andreasen NC, Endicott J, Spitzer RL, Winokur G (1977). The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiat 34: 1229–1235.
Arvanitis LA, Miller BG (1997). Multiple fixed doses of ‘seroquel’ (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The seroquel trial 13 study group. Biol Psychiat 42: 233–246.
Breier A, Su T-P, Saunders R, Carson RE, Kolachana BS, De Bartolomeis A et al (1997). Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 94: 2569–2574.
Bruggeman RM, Heeringa M, Westerink BHC, Timmerman W (2000). Combined 5-HT2/D2 receptor blockade inhibits the firing rate of SNR neurons in the rat brain. Prog Neuro-Psychopharm Biol Psychiat 24: 579–593.
Buchsbaum MS, Christian BT, Lehrer DS, Mukherjee J, Siu B, Mantil J (2004). D2/D3 dopamine receptor binding in the thalamus of medication-naïve schizophrenics. Int J Neuropsychopharmacol 7: S430.
Chiodo LA, Bunney BS (1983). Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci 3: 1607–1619.
Christian BT, Narayanan T, Shi B, Morris ED, Mantil J, Mukherjee J (2004). Measuring the in vivo binding parameters of [18F]-fallypride in monkeys using a PET multiple-injection protocol. J Cereb Blood Flow Metab 24: 309–322.
Cobb WS, Abercrombie ED (2003). Differential regulation of somatodendritic and nerve terminal dopamine release by serotonergic innervation of substantia nigra. J Neurochem 84: 576–584.
Conley RR, Kelly DL, Nelson MW, Richardson CM, Feldman S, Benham R et al (2005). Risperidone, quetiapine and fluphenazine in the treatment of patients with therapy-refractory schizophrenia. Clin Neuropharmacol 28: 163–168.
Crocker AD, Hemsley KM (2001). An animal model of extrapyramidal side effects induced by antipsychotic drugs: relationship with a D2 dopamine receptor occupancy. Prog Neuro-Psychopharm Biol Psychiat 25: 573–590.
Damask SP, Bovenkerk KA, De la Pena G, Hoversten KM, Peters DB, Valentine AM et al (1996). Differential effects of clozapine and haloperidol dopamine receptor mRNA expression in rat striatum and cortex. Brain Res Mol Brain Res 41: 241–249.
Davis JM, Chen N, Glick ID (2003). A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiat 60: 553–564.
Delforge J, Bottlaender M, Loc'h C, Dolle F, Syrota A (2001). Parametric images of the extrastriatal d2 receptor density obtained using a high-affinity ligand (FLB 457) and a double-saturation method. J Cereb Blood Flow Metab 21: 1493–1503.
Descarries L, Watkins KC, Garcia S, Bosler O, Doucet G (1996). Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: a quantitative autoradiographic and immunocytochemical analysis. J Comp Neurol 375: 167–186.
American Psychiatric Association 1994. Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (DSM-IV). American Psychiatric Association: Washington, DC.
Double KL, Crocker AS (1995). Dopamine receptors in the substantia nigra are involved in the regulation of muscle tone. Proc Natl Acad Sci USA 92: 1669–1673.
Erlandsson K, Bressan RA, Mulligan RS, Ell PJ, Cunningham VJ, Pilowsky LS (2003). Analysis of D2 dopamine receptor occupancy with quantitative SPET using the high-affinity ligand [123I]epidepride: resolving conflicting findings. NeuroImage 19: 1205–1214.
Fann WE, Stafford JR, Malone RL, Frost Jr JD, Richman BW (1977). Clinical research techniques in tardive dyskinesia. Am J Psychiat 134: 759–762.
Farde L, Nordstrom NS, Halldin C, Sedvall G (1994). D1-, D2- and 5-HT2-receptor occupancy in clozapine-treated patents. J Clin Psychiat 9 (Suppl BJ): 67–69.
Farde L, Wiesel FA, Stone-Elander S, Halldin C, Nordstrom AL, Hall H et al (1990). D2 dopamine receptors in neuroleptics-naïve schizophrenic patients: a positron emission tomography study with [11C] raclopride. Arch Gen Psychiat 47: 213–219.
First MB, Spitzer RL, Gibbon M, Williams JBW 1996. Structured Clinical Interview for Axis I DSM-IV Disorders—Patient Edition (with Psychotic Screen) (SCID-I/P) (Version 2.0). Biometrics Research Department, New York State Psychiatric Institute: New York.
Florijn WJ, Tarazi FI, Creese I (1997). Dopamine receptor subtypes: differential regulation after 8 months treatment with antipsychotic drugs. J Pharm Exp Ther 280: 561–569.
Fujita M, Selbyl JP, Verhoeff PILG, Ichise M, Baldwin RM, Zoghbi SS et al (1999). Kinetic and equilibrium analyses of [123I]epidepride binding to striatal and extrastriatal dopamine D2 receptors. Synapse 34: 290–304.
Garris PA, Ciolkowski EL, Pastore P, Wightman RM (1994). Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14: 6084–6093.
Garris PA, Wightman RM (1994). Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci 14: 442–450.
Gefvert O, Bergström M, Långström, Lundberg T, Lindström L, Yates R (1998). Time course of central nervous dopamine-D2 and 5-HT2 receptor blockade and plasma drug concentrations after discontinuation of quetiapine (Seroquel®) in patients with schizophrenia. Psychopharmacology 135: 119–126.
Gerhardt GA, Cass WA, Huettl P, Brock S, Zhang ZM, Gash DM (1999). GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res 817: 163–171.
Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM (2002). Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem 80: 168–177.
Goldstein JM, Litwin LC, Sutton EB, Malick JB (1993). Seroquel: electrophysiological profile of a potential atypical antipsychotic. Psychopharmacology (Berl) 112: 293–298.
Grunder G, Landvogt C, Vernaleken, Buchholz H-G, Ondracek J, Siessmeier (2006). The striatal and extrastriatal D2/3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology 31: 1027–1035.
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997). Parametric imaging of ligand–receptor binding in PET using a simplified reference region model. NeuroImage 6: 279–287.
Hietala J, Syvalahti E, Vuorio K, Nagren K, Lehikoinen P, Ruotsalainen U et al (1994). Striatal D2 dopamine receptor characteristics in neuroleptics- naïve schizophrenic patients studied with positron emission tomography. Arch Gen Psychiat 51: 116–123.
Ichikawa J, Dai J, Meltzer HY (2002). Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5HT1A receptor agonism. Brain Res 956: 349–357.
Janowsky A, Neve KA, Kinzie JM, Taylor B, dePaulis T, Belknap JK (1992). Extrastriatal dopamine D2 receptors: distribution pharmacological characterization and region-specific regulation by clozapine. J Pharm Exp Ther 261: 1282–1290.
Kane JM, Marder SR, Schooler NR, Wirshing WC, Umbricht D, Baker RW (2001). Clozapine and haloperidol in moderately refractory schizophrenia. Arch Gen Psychiat 58: 965–972.
Kapur S, Remington G, Jones C, Wilson A, DaSilva J, Houle S et al (1996). High levels of dopamine D2 receptor occupancy with low-dose haloperidol treatment: a PET study. Am J Psychiat 153: 948–950.
Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P (2000). A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiat 57: 553–559.
Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA et al (1998). 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiat 155: 921–928.
Kawagoe KT, Garris PA, Wiedmann DJ, Wightman RM (1992). Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience 51: 55–64.
Kessler R, Ansari MS, Li R, Lee M, Schmidt D, Dawant B et al (2002). Occupancy of cortical and substantia nigra DA D2 receptors by typical and atypical antipsychotic drugs. NeuroImage 16: S9.
Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B et al (2005). Occupancy of striatal and extrastriatal DA D2/3 receptors by olanzapine and haloperidol. Neuropsychopharmacology 30: 2283–2289.
Kessler RM, Ellis Jr JR, Eden M (1984). Analysis of emission tomographic scan data: limitations imposed by resolution and background. J Comput Assist Tomogr 8: 514–522.
Kessler RM, Mason NS, Jones C, Ansari MS, Manning RF, Price RR (2000). [18F]N-allyl-5-fluoropropylepidepride (fallypride): radiation dosimetry, quantification of striatal and extrastriatal dopamine receptors in man. NeuroImage 11: S32.
Kessler RM, Meltzer HY (2002). Regional selectivity in clozapine treatment. Am J Psy 159: 1064–1065.
Khan ZU, Mrzljak L, Gutierrez A, DeLa Calle A, Goldman-Rakic PS (1998). Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Scad Sci Neurobiol 95: 7731–7736.
Kuroki T, Meltzer HY, Ichikawa J (1999). Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharm Exp Ther 288: 774–781.
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ et al (1996). Comparison of methods of analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 16: 42–52.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. NeuroImage 4 (3 Part 1): 153–158.
Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA et al (1993). Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA 90: 8861–8865.
Lidow MS, Goldman-Rakic PS (1997). Differential regulation of D2 and D4 dopamine receptor mRNAs in the primate cerebral cortex vs neostriatum: effects of chronic treatment with typical and atypical antipsychotic drugs. J Pharmacol Exp Therapeut 283: 939–946.
Maes F, Collignon A, Vandermuele D, Marchal G, Suetens P (1997). Multimodality image registration by maximization of mutual information. IEEE Trans Med Imag 16: 187–198.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al (2001). Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Acurracy and precision of D (2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21: 1034–1057.
Meltzer HY, Matsubara S, Lee J-C (1989). Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Therapeut 251: 238–246.
Meltzer HY (1992). The mechanism of action of clozapine in relation to its clinical advantage. In: Meltzer HY (ed). Novel Antipsychotic Drugs. Raven Press: New York. pp 1–13.
Meltzer HY. (1999). The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 21: 106S–115S.
Meltzer HY, Arvanitis L, Bauer D, Rein W (2004). Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiat 161: 975–984.
Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M et al (2002). Brain images of 18F-fallypride in normal volunteers: blood analysis, distribution, test–retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse 46: 170–188.
Nordstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C et al (1993). Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects—a double-blind PET study of schizophrenic patients. Biol Psychiat 33: 227–235.
Nordstrom AL, Nyberg S, Olsson H, Farde L (1998). Positron emission tomography finding of a high striatal D2 receptor occupancy in olanzapine-treated patients. Arch Gen Psychiat 55: 283–284.
Olsson H, Farde L (2001). Potentials and pitfalls using high affinity radioligands in PET and SPET determinations on regional drug induced D2 receptor occupancy—a simulation study based on experimental data. NeuroImage 14: 945–946.
Olsson H, Halldin C, Farde L (2004). Differentiation of extrastiatal dopamine D2 receptor density and affinity in the human brain using PET. NeuroImage 22: 794–803.
Olsson H, Halldin C, Swahn CG, Farde L (1999). Quantification of [11C] FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab 10: 1164–1173.
Pilowsky LS, Mulligan RS, Acton PD, Ell PJ, Costa D, Kerwin RW (1997). Limbic selectivity of clozapine. Lancet 350: 490–491.
Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B et al (2006). Amphetamine induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology 31: 1016–1026.
Rieck RW, Ansari MS, Whetsell Jr WO, Deutch AY, Kessler RM (2004). Distribution of dopamine D2-like receptors in the human thalamus: autoradiographic and PET studies. Neuropsychopharmacology 29: 362–372.
Robertson GS, Fibiger HC (1992). Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience 46: 315–328.
Robertson GS, Matsumura H, Fibiger HC (1994). Induction patterns of fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther 271: 1058–1066.
Roth BL, Sheffler D, Potkin SG (2003). Atypical antipsychotic drug actions: unitary or multiple mechanisms for ‘atypicality’? Clin Neurosci Res 3: 108–117.
Roth BL, Tandra S, Burgess LH, Siblry DR, Meltzer HY (1995). D4 dopamine receptor binding affinity does not distinquish between typical and atypical antipsychotic drugs. Psychopharmacology 120: 365–368.
Schaltenbrand G, Wahren W (1977). Atlas for Stereotaxy of the Human Brain. Yearbook Medical Publisher Inc.: Chicago.
Schotte A, Janssen PFM, Gommeren WM, Lyuten WHML, Van Gompel P, Lesage AS et al (1996). Risperidone compared with new and reference antipsychotic drugs: in vigor and in vivo receptor binding. Psychopharmacology 124: 57–73.
Seeman P, Tallerico T (1998). Antipsychotic drugs which elicit little or no Parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiat 3: 123–134.
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998). Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18: 2697–2708.
Siessmeier T, Zhou Y, Buchholz HG, Landvogt C, Vernaleken I, Piel M et al (2005). Parametric mapping of binding in human brain of D2 receptor ligands of different affinities. J Nucl Med 46: 964–972.
Slifstein M, Hwang D-R, Huang Y, Guo NN, Sudo Y, Narendran R et al (2004). In vivo affinity of [18F]fallypride for striatal and extrastriatal dopamine D2 receptors in nonhuman primates. Psychopharmacology 175: 274–286.
Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS (1994). D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA Neurobiol 91: 5720–5724.
Sorenson SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK et al (1993). Characterization of the 5-HT2 receptor antagonist MLD 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharm Exp Ther 266: 684–691.
Stephenson CME, Bigliani V, Jones HM, Mulligan RS, Acton PD, Visvikis D et al (2000). Striatal and extra-striatal D2/D3 dopamine receptor occupancy by quetiapine in vivo. Br J Psychiat 177: 408–415.
Svensson TH (2003). Adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuro-Psychopharmacol Biol Psychiat 27: 1145–1158.
Talvik M, Nordstrom AL, Olsson H, Halldin C, Farde L (2003). Decreased thalamic D2/D3 receptor binding in drug-naïve patients with schizophrenia: a PET study with [11C]FLB 457. Int J Neuropsychopharmacol 6: 361–370.
Talvik M, Nordström AL, Nyberg S, Olsson H, Halldin C, Lars F (2001). No support for regional selectivity in clozapine-treated patients: a PET study with [11C]raclopride and [11C] FLB 457. Am J Psychiat 158: 926–930.
Tauscher-Wisniewski S, Kapur S, Tauscher J, Jones C, Daskalakis ZJ, Papatheodorou G et al (2002). Quetiapine: an effective antipsychotic in first-episode schizophrenia despite only transiently high dopamine-2 receptor blockade. J Clin Psychiat 63: 992–997.
Tuppurainen H, Kuikka J, Viinamaki H, Husso-Saastamoinen M, Bergstrom K, Tiihonen J (2003). Extrastriatal dopamine D 2/3 receptor density and distribution in drug-naïve schizophrenic patients. Mol Psychiat 8: 453–455.
Vahid-Ansari F, Nakabeppu Y, Robertson GS (1996). Contrasting effects of chronic clozapine, Seroquel (TM) (ICI 204,636) and haloperidol administration of delta FosB-like immunoreactivity in the rodent forebrain. Eur J Neurosci 8: 927–936.
Venton BJ, Zhang H, Garris PA, Phillips PEM, Sulzer D, Wightman RM (2003). Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem 87: 1284–1295.
Wells III WM, Viola P, Atsumi H, Nakajima S, Kikinis R (1996). Multimodal volume registration by maximization of mutual information. Med Imag Anal 1: 35–51.
Xiberas X, Marinot JL, Mallet L, Artiges E, Loc'h C, Mazière B et al (2000). Extrastriatal and striatal D2 dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiat 179: 503–508.
Yamamoto BK, Cooperman MA (1994). Differential effects of chronic antipsychotic drug treatment on extracellular glutamate and dopamine concentrations. J Neurosci 14: 4159–4166.
Yasuno F, Suhara T, Okubo Y, Sudo Y, Inoue M, Ichimiya T et al (2004). Low dopamine d(2) receptor binding in subregions of the thalamus in schizophrenia. Am J Psychiat 161: 1016–1022.
Youngren KD, Inglis FM, Pivirotto PH, Jedema HP, Bradberry CW, Goldman-Rakic PS et al (1999). Clozapine preferentially increases dopamine release in the rhesus monkey prefrontal cortex compared with the caudate nucleus. Neuropsychopharmacology 20: 403–412.
Acknowledgements
This work was supported by NIMH Grant number 1R01MH60898, by funding from Astra Zeneca, from the William K Warren Medical Research Foundation, the Ritter Foundation, and Centerstone Mental Health Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kessler, R., Ansari, M., Riccardi, P. et al. Occupancy of Striatal and Extrastriatal Dopamine D2 Receptors by Clozapine and Quetiapine. Neuropsychopharmacol 31, 1991–2001 (2006). https://doi.org/10.1038/sj.npp.1301108
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301108
Keywords
This article is cited by
-
Neuroimaging glutamatergic mechanisms differentiating antipsychotic treatment-response
Scientific Reports (2023)
-
Machine Learning algorithm unveils glutamatergic alterations in the post-mortem schizophrenia brain
Schizophrenia (2022)
-
The relationship between excitement symptom severity and extrastriatal dopamine D2/3 receptor availability in patients with schizophrenia: a high-resolution PET study with [18F]fallypride
European Archives of Psychiatry and Clinical Neuroscience (2018)
-
First- and second-generation antipsychotic drug treatment and subcortical brain morphology in schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2016)
-
Psychosis in Parkinson’s Disease: Epidemiology, Pathophysiology, and Management
Drugs (2016)