Abstract
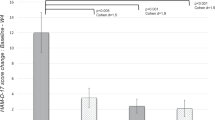
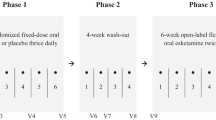
Approximately one-third of persons with depression do not respond to antidepressant monotherapy. Studies suggest that atypical antipsychotic augmentation may benefit these patients. We investigated the longer-term efficacy of risperidone augmentation of serotonin-selective reuptake inhibitor treatment for resistant depression. In 57 in- and outpatient centers in three countries, we conducted a three-phase study with 4–6 weeks of open-label citalopram monotherapy, 4–6 weeks of open-label risperidone augmentation, and a 24-week double-blind, placebo-controlled discontinuation phase. A total of 489 patients with major depressive disorder and 1–3 documented treatment failures entered the citalopram monotherapy phase (20–60 mg/day). Patients with <50% reduction in HAM-D-17 scores entered the risperidone augmentation phase (0.25–2.0 mg/day). Patients with HAM-D-17⩽7 or CGI-S⩽2 were randomized to risperidone or placebo augmentation. The primary outcome was time to relapse during the double-blind phase. During citalopram monotherapy, 434 patients had <50% HAM-D-17 reduction; 299 (68.9%) were fully nonresponsive (<25% reduction) and 135 were partially nonresponsive (25–49% reduction). Of the 386 nonresponders who entered the augmentation phase, 243 remitted and 241 entered the double-blind phase. Median time to relapse was 102 days with risperidone augmentation and 85 days with placebo (NS); relapse rates were 53.3 and 54.6%, respectively. In a post hoc analysis of patients fully nonresponsive to citalopram monotherapy, median time to relapse was 97 days with risperidone augmentation and 56 with placebo (p=0.05); relapse rates were 56.1 and 64.1%, respectively (p⩽0.05). Open-label risperidone augmentation substantially enhanced response in treatment-resistant patients, but the longer-term benefits of augmentation were not demonstrated in this study.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adson DE, Kushner MG, Eiben KM, Schulz SC (2004). Preliminary experience with adjunctive quetiapine in patients receiving selective serotonin reuptake inhibitors. Depress Anxiety 19: 121–126.
American Psychiatric Association (2000). Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiat 157: 1–45.
Amsterdam JD, Hornig-Rohan M (1996). Treatment algorithms in treatment-resistant depression. Psychiatr Clin N Am 19: 371–386.
Ananth J (1998). Treatment-resistant depression. Psychother Psychosom 67: 61–70.
Barnes TR (1989). A rating scale for drug-induced akathisia. Br J Psychiat 154: 672–676.
Bodkin JA, Lasser RA, Wines Jr JD, Gardner DM, Baldessarini RJ (1997). Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiat 58: 137–145.
De Jonghe F, Hendricksen M, van Aalst J, Kool S, Peen V, Van R et al (2004). Psychotherapy alone and combined with pharmacotherapy in the treatment of depression. Br J Psychiat 185: 37–45.
Einarson TR (2004). Evidence based review of escitalopram in treating major depressive disorder in primary care. Int Clin Psychopharmacol 19: 305–310.
Fava GA, Ruini C, Rafanelli C, Finos L, Conti S, Grandi S (2004). Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiat 161: 1872–1876.
Fava M (2001). Augmentation and combination strategies in treatment-resistant depression. J Clin Psychiat 62(Suppl 18): 4–11.
Fava M, Davidson KG (1996). Definition and epidemiology of treatment-resistant depression. Psychiatr Clin N Am 19: 179–2000.
Figiel GS, Epstein C, McDonald WM, Amazon-Leece J, Figiel L, Saldivia A et al (1998). The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiat Clin Neurosci 10: 2025.
Gomez Gomez JM, Teixido Perramon C (2000). Combined treatment with venlafaxine and tricyclic antidepressants in depressed patients who had partial response to clomipramine or imipramine: initial findings. J Clin Psychiat 61: 285–289.
Greden JF (2001). The burden of disease for treatment-resistant depression. J Clin Psychiat 62(Suppl 16): 26–31.
Guy W (1976). ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. US Department of Health, Education, and Welfare: Washington, DC. pp 218–222.
Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiat 23: 56–62.
Joffe RT, Singer W, Levitt AJ, MacDonald C (1993). A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiat 50: 387–393.
Keller MB (2005). Issues in treatment-resistant depression. J Clin Psychiat 66(Suppl 8): 5–12.
McGrath PJ, Stewart JW, Nunes EV, Ocepek-Welikson K, Rabkin JG, Quitkin FM et al (1993). A double-blind crossover trial of imipramine and phenelzine for outpatients with treatment-refractory depression. Am J Psychiat 150: 118–123.
Montgomery SA, Asberg M (1979). A new depression scale designed to be sensitive to change. Br J Psychiat 134: 382–389; 592.
Nierenberg AA, Amsterdam JD (1990). Treatment-resistant depression: definition and treatment approaches. J Clin Psychiat 51(Suppl): 39–47; discussion 48–50.
Nierenberg AA, DeCecco LM (2001). Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiat 62(Suppl 16): 5–9.
Ostroff RB, Nelson JC (1999). Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiat 60: 256–259.
Papakostas GI, Petersen TJ, Nierenberg AA, Murakami JL, Alpert JE, Rosenbaum JF et al (2004). Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiat 65: 217–221.
Rapaport MH, Bose A, Zheng H (2004). Escitalopram continuation treatment prevents relapse of depressive episodes. J Clin Psychiat 65: 44–49.
Robert P, Montgomery SA (1995). Citalopram in doses of 20–60 mg is effective in depression relapse prevention: a placebo-controlled 6 month study. Int Clin Psychopharmacol 10(Suppl 1): 29–35.
Robins LN, Regier DA (1991). Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press: New York.
Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C et al (2000). Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiat 47: 276–286.
Shelton RC, Tollefson GD, Tohen M, Stahl S, Gannon KS, Jacobs TG et al (2001). A novel augmentation strategy for treating resistant major depression. Am J Psychiat 158: 131–134.
Shelton RC, Williamson DJ, Corya SA, Sanger TM, Van Campen LE, Case M et al (2005). Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiat 66: 1289–1297.
Simpson GM, Angus JW (1970). A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212: 11–19.
Solomon DA, Leon AC, Endicott J, Mueller TI, Coryell W, Shea MT et al (2004). Psychosocial impairment and recurrence of major depression. Compr Psychiat 45: 423–430.
Thase ME, Feighner JP, Lydiard RB (2001). Citalopram treatment of fluoxetine nonresponders. J Clin Psychiat 62: 683–687.
Thase ME, Rush AJ (1995). Treatment resistant depression. In: Bloom FE, Kupfer DJ (eds). Neuropsychopharmacology: The Fourth Generation of Progress. Raven Press: New York. pp 1081–1097.
Thase ME, Rush AJ (1997). When at first you don't succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiat 58(Suppl 13): 23–29.
Tranter R, O'Donovan C, Chandarana P, Kennedy S (2002). Prevalence and outcome of partial remission in depression. J Psychiat Neurosci 27: 241–247.
Wahlund B, von Rosen D (2003). ECT of major depressed patients in relation to biological and clinical variables: a brief overview. Neuropsychopharmacology 28(Suppl 1): S21–S26.
Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG et al (1996). Cross-national epidemiology of major depression and bipolar disorder. JAMA 276: 293–299.
Acknowledgements
This research was funded by Medical Affairs, Janssen Pharmaceutica, LP. MH 61757-A2, 1R21 AT002751-01, 1R01 MH73765-01A1, Cedars-Sinai GCRC Grant RR00425 and The Polier Endowed Chair for Schizophrenia and Related Disorders were supported MHR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rapaport, M., Gharabawi, G., Canuso, C. et al. Effects of Risperidone Augmentation in Patients with Treatment-Resistant Depression: Results of Open-Label Treatment Followed by Double-Blind Continuation. Neuropsychopharmacol 31, 2505–2513 (2006). https://doi.org/10.1038/sj.npp.1301113
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301113
Keywords
This article is cited by
-
Efficacy and Safety of Olanzapine/Fluoxetine Combination vs Fluoxetine Monotherapy Following Successful Combination Therapy of Treatment-Resistant Major Depressive Disorder
Neuropsychopharmacology (2014)
-
Augmentation Treatments with Second-generation Antipsychotics to Antidepressants in Treatment-resistant Depression
CNS Drugs (2013)
-
Molecular mechanisms underlying synergistic effects of SSRI–antipsychotic augmentation in treatment of negative symptoms in schizophrenia
Journal of Neural Transmission (2009)
-
Potentiation of excitatory serotonergic responses by MK-801 in the medial prefrontal cortex
Naunyn-Schmiedeberg's Archives of Pharmacology (2009)
-
Augmentation effect of combination therapy of aripiprazole and antidepressants on forced swimming test in mice
Psychopharmacology (2009)