Abstract
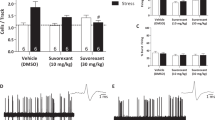
Antipsychotic drugs alter the activity of dopamine neurons in the ventral tegmental area (A10) and substantia nigra pars compacta (A9). As there is a dense projection of orexin neurons from the lateral hypothalamus to A10 dopaminergic neurons, and some antipsychotics have been shown to increase the expression of c-fos in orexin-containing cells in the hypothalamus, we hypothesized that stimulation of orexin receptors plays a role in the effects of antipsychotics on the activity of A9 and A10 dopamine cells. Single-unit recordings in anesthetized rats demonstrated the central effects of the selective orexin-1 receptor antagonist SB-334867 (2 mg/kg, intravenous), as it reversed the excitatory effects of orexin-A administration (6 μg, intracerebroventricular) on the activity of locus coeruleus (LC) cells. Recordings from midbrain dopamine neurons showed that acute administration of SB-334867 alone did not alter the number of spontaneously active A9 or A10 cells, but did reverse: (1) the increase in the number of spontaneously active A9 and/or A10 dopamine cells caused by the acute administration of haloperidol (1 mg/kg, subcutaneous) or olanzapine (10 mg/kg, s.c.) and (2) the decrease in the number of spontaneously active A9 and/or A10 dopamine cells caused by the chronic administration of haloperidol (1 mg/kg/day × 21 days, s.c.) or olanzapine (10 mg/kg/day × 21 days, s.c.). However, SB-334867 did not block a different electrophysiological effect of olanzapine, as it did not block the olanzapine-induced activation of LC cells. These results indicate that activation of orexin-1 receptors plays an important role on the effects of antipsychotic drugs on dopamine neuronal activity and may play an important role in the clinical effects of antipsychotic drugs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Berridge KC, Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28: 309–369.
Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006). Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49: 589–601.
Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR et al (2000). Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci 20: 7760–7765.
Bunney BS, Grace AA (1978). Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci 23: 1715–1728.
Bunney BS, Walters JR, Roth RH, Aghajanian GK (1973). Dopaminergic neurons: effects of antipsychotic drugs and amphetamine on single cell activity. J Pharm Exp Ther 185: 560–571.
Carr DB, Sesack SR (2000). Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 20: 3864–3873.
Chiodo LA, Bunney BS (1983). Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci 3: 1607–1619.
Datla KP, Ahier RG, Young AM, Gray JA, Joseph MH (2002). Conditioned appetitive stimulus increases extracellular dopamine in the nucleus accumbens of the rat. Eur J Neurosci 16: 1987–1993.
Dawe GS, Huff KD, Vandergriff JL, Sharp T, O'Neill MJ, Rasmussen K (2001). Olanzapine activates the rat locus coeruleus: in vivo electrophysiology and c-Fos immunoreactivity. Biol Psychiatry 50: 510–520.
Deutch AY, Jackson LL, Bubser M (2005). Disruption of orexin/hypocretin signaling blocks the effects of clozapine on the prefrontal cortex. Schiz Bull 31: 510.
Espana RA, Valentino RJ, Berridge CW (2003). Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience 121: 201–217.
Fadel J, Bubser M, Deutch AY (2002). Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci 22: 6742–6746.
Fadel J, Deutch AY (2002). Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience 111: 379–387.
Floresco SB, Todd CL, Grace AA (2001). Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 21: 4915–4922.
Floresco SB, West AR, Ash B, Moore H, Grace AA (2003). Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6: 968–973.
Georges F, Aston-Jones G (2002). Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22: 5173–5187.
Graham AW, Aghajanian GK (1971). Effects of amphetamine on single cell activity in a catecholamine nucleus, the locus coeruleus. Nature 234: 100–102.
Gueudet C, Santucci V, Soubrie P, Le Fur G (1999). Blockade of neurokinin3 receptors antagonizes drug-induced population response and depolarization block of midbrain dopamine neurons in guinea pigs. Synapse 33: 71–79.
Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S et al (1999). Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 96: 10911–10916.
Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA (2001). Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103: 777–797.
Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE (2003). Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci 23: 7–11.
Lodge DJ, Grace AA (2006). The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci USA 103: 5167–5172.
Meltzer HY, Arvanitis L, Bauer D, Rein W, Meta-Trial Study Group (2004). Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 161: 975–984.
Murai Y, Akaike T (2005). Orexins cause depolarization via nonselective cationic and K+ channels in isolated locus coeruleus neurons. Neurosci Res 51: 55–65.
Phillipson OT (1979). Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol 187: 117–143.
Piper DC, Upton N, Smith MI, Hunter AJ (2000). The novel brain neuropeptide, orexin-A, modulates the sleep–wake cycle of rats. Eur J Neurosci 12: 726–730.
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H et al (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585.
Schultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27.
Seager MA, Huff KD, Barth VN, Phebus LA, Rasmussen K (2004). Fluoxetine administration potentiates the effect of olanzapine on locus coeruleus neuronal activity. Biol Psychiatry 55: 1103–1109.
Sesack SR, Carr DB (2002). Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav 77: 513–517.
Sesack SR, Pickel VM (1992). Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol 320: 145–160.
Smart D, Jerman JC, Brough SJ, Neville WA, Jewitt F, Porter RA (2000). The hypocretins are weak agonists at recombinant human orexin-1 and orexin-2 receptors. Br J Pharmacol 129: 1289–1291.
Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F et al (1999). Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol 128: 1–3.
Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA et al (2001). SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol 132: 1179–1182.
Soffin EM, Evans ML, Gill CH, Harries MH, Benham CD, Davies CH (2002). SB-334867-A antagonises orexin mediated excitation in the locus coeruleus. Neuropharmacology 42: 127–133.
Stockton ME, Rasmussen K (1996). Olanzapine, a novel atypical antipsychotic, reverses d-amphetamine-induced inhibition of midbrain dopamine cells. Psychopharmacol 124: 50–56.
van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB (1998). Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci 18: 7962–7971.
Vittoz NM, Berridge CW (2006). Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology 31: 384–395.
White FJ, Wang RY (1983). Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science 221: 1054–1057.
Acknowledgements
We acknowledge the contributions of Dr Laura Nisenbaum to the completion of this manuscript. We are employees and stock holders of Eli Lilly & Co.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rasmussen, K., Hsu, MA. & Yang, Y. The Orexin-1 Receptor Antagonist SB-334867 Blocks the Effects of Antipsychotics on the Activity of A9 and A10 Dopamine Neurons: Implications for Antipsychotic Therapy. Neuropsychopharmacol 32, 786–792 (2007). https://doi.org/10.1038/sj.npp.1301239
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301239
Keywords
This article is cited by
-
Therapeutic effects of orexin-A in sepsis-associated encephalopathy in mice
Journal of Neuroinflammation (2024)
-
Orexin A alleviates neuroinflammation via OXR2/CaMKKβ/AMPK signaling pathway after ICH in mice
Journal of Neuroinflammation (2020)
-
Orexin Directly Enhances the Excitability of Globus Pallidus Internus Neurons in Rat by Co-activating OX1 and OX2 Receptors
Neuroscience Bulletin (2017)
-
Application of nano SnO2 as a green and recyclable catalyst for the synthesis of 2-aryl or alkylbenzoxazole derivatives under ambient temperature
Journal of Chemical Sciences (2014)
-
Eco-friendly synthesis of 2-substituted benzothiazoles catalyzed by silica sulfuric acid
Research on Chemical Intermediates (2013)