Abstract
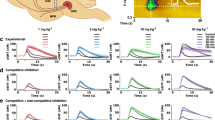
The therapeutic effects of chronic selective serotonin reuptake inhibitors (SSRIs) are well documented, yet the elementary behavioral processes that are affected by such treatment have not been fully investigated. We report here the effects of chronic fluoxetine treatment and genetic deletion of the serotonin transporter (SERT) on food reinforced behavior in three paradigms: the progressive ratio operant task, the concurrent choice operant task, and the Pavlovian-to-Instrumental transfer task. We consistently find that chronic pharmacological blockade or genetic deletion of SERT result in similar behavioral consequences: reduced operant responding for natural reward. This is in line with previous studies reporting declines in operant responding for drugs and intracranial self-stimulation with fluoxetine treatment, suggesting that the effect of SERT blockade can be generalized to different reward types. Detailed analyses of behavioral parameters indicate that this reduction in operant responding affect both goal-directed and non-goal-directed behaviors without affecting the Pavlovian cue-triggered excessive operant responding. In addition, both pharmacological and genetic manipulations reduce locomotor activity in the open field novel environment. Our data contrast with the effect of dopamine in increasing operant responding for natural reward specifically in goal-directed behaviors and in increasing Pavlovian cue-triggered excessive operant responding. Serotonin and dopamine have been proposed to serve opposing functions in motivational processes. Our data suggest that their interactions do not result in simple opponency. The fact that pharmacological blockade and genetic deletion of SERT have similar behavioral consequences reinforces the utility of the SERT null mice for investigation of the mechanisms underlying chronic SSRIs treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aberman JE, Salamone JD (1999). Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92: 545–552.
Aberman JE, Ward SJ, Salamone JD (1998). Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav 61: 341–348.
Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306: 879–881.
Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL (2001). Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berlin) 155: 18–26.
Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A et al (1998). Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (‘Ecstasy’) in serotonin transporter-deficient mice. Mol Pharmacol 53: 649–655.
Bergqvist PB, Bouchard C, Blier P (1999). Effect of long-term administration of antidepressant treatments on serotonin release in brain regions involved in obsessive-compulsive disorder. Biol Psychiatry 45: 164–174.
Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiebot M (1999). Serotonin and tolerance to delay of reward in rats. Psychopharmacology (Berlin) 146: 400–412.
Bubar MJ, McMahon LR, De Deurwaerdere P, Spampinato U, Cunningham KA (2003). Selective serotonin reuptake inhibitors enhance cocaine-induced locomotor activity and dopamine release in the nucleus accumbens. Neuropharmacology 44: 342–353.
Cagniard B, Balsam PD, Brunner D, Zhuang X (2006). Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology 31: 1362–1370.
Cameron DL, Williams JT (1995). Opposing roles for dopamine and serotonin at presynaptic receptors in the ventral tegmental area. Clin Exp Pharmacol Physiol 22: 841–845.
Carroll ME, Lac ST, Asencio M, Kragh R (1990). Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav 35: 237–244.
Correa M, Carlson BB, Wisniecki A, Salamone JD (2002). Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res 137: 179–187.
Cousins MS, Atherton A, Turner L, Salamone JD (1996). Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res 74: 189–197.
Cousins MS, Salamone JD (1994). Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav 49: 85–91.
Cousins MS, Wei W, Salamone JD (1994). Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology (Berlin) 116: 529–537.
Cryan JF, Valentino RJ, Lucki I (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29: 547–569.
Daw ND, Kakade S, Dayan P (2002). Opponent interactions between serotonin and dopamine. Neural Netw 15: 603–616.
Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999). Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283: 397–401.
Glatz AC, Ehrlich M, Bae RS, Clarke MJ, Quinlan PA, Brown EC et al (2002). Inhibition of cocaine self-administration by fluoxetine or D-fenfluramine combined with phentermine. Pharmacol Biochem Behav 71: 197–204.
Halford JC, Harrold JA, Lawton CL, Blundell JE (2005). Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity. Curr Drug Targets 6: 201–213.
Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD (1999). Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav 64: 21–27.
Harrison AA, Liem YT, Markou A (2001). Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology 25: 55–71.
Harrison AA, Markou A (2001). Serotonergic manipulations both potentiate and reduce brain stimulation reward in rats: involvement of serotonin-1A receptors. J Pharmacol Exp Ther 297: 316–325.
Hirschfeld RM (2000). History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 61 (Suppl 6): 4–6.
Hodos W (1961). Progressive ratio as a measure of reward strength. Science 134: 943–944.
Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN (2003c). Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav 2: 365–380.
Holmes A, Murphy DL, Crawley JN (2002a). Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berlin) 161: 160–167.
Holmes A, Murphy DL, Crawley JN (2003a). Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry 54: 953–959.
Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL (2003b). Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology 28: 2077–2088.
Holmes A, Yang RJ, Murphy DL, Crawley JN (2002b). Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology 27: 914–923.
Hubbell CL, Marglin SH, Spitalnic SJ, Abelson ML, Wild KD, Reid LD (1991). Opioidergic, serotonergic, and dopaminergic manipulations and rats' intake of a sweetened alcoholic beverage. Alcohol 8: 355–367.
Ioannides-Demos LL, Proietto J, McNeil JJ (2005). Pharmacotherapy for obesity. Drugs 65: 1391–1418.
Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD (2004). Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res 151: 83–91.
Joel D, Ben-Amir E, Doljansky J, Flaisher S (2004). ‘Compulsive’ lever-pressing in rats is attenuated by the serotonin re-uptake inhibitors paroxetine and fluvoxamine but not by the tricyclic antidepressant desipramine or the anxiolytic diazepam. Behav Pharmacol 15: 241–252.
Lee K, Kornetsky C (1998). Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav 60: 539–544.
Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH et al (2003). Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry 54: 960–971.
Luciana M, Collins PF, Depue RA (1998). Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cereb Cortex 8: 218–226.
Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD (2005). Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci 21: 1749–1757.
Nowend KL, Arizzi M, Carlson BB, Salamone JD (2001). D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav 69: 373–382.
Owens MJ (2004). Selectivity of antidepressants: from the monoamine hypothesis of depression to the SSRI revolution and beyond. J Clin Psychiatry 65 (Suppl 4): 5–10.
Richardson NR, Roberts DC (1991). Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci 49: 833–840.
Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE (2002). Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berlin) 160: 371–380.
Salamone JD, Kurth P, McCullough LD, Sokolowski JD (1995). The effects of nucleus accumbens dopamine depletions on continuously reinforced operant responding: contrasts with the effects of extinction. Pharmacol Biochem Behav 50: 437–443.
Salamone JD, Wisniecki A, Carlson BB, Correa M (2001). Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience 105: 863–870.
Sokolowski JD, Salamone JD (1998). The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav 59: 557–566.
Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R et al (1998). Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA 95: 7699–7704.
Tella SR (1995). Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav 51: 687–692.
Wilson AW, Costall B, Neill JC (2000). Manipulation of operant responding for an ethanol-paired conditioned stimulus in the rat by pharmacological alteration of the serotonergic system. J Psychopharmacol 14: 340–346.
Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004). Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 29: 1331–1343.
Wyvell CL, Berridge KC (2000). Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward ‘wanting’ without enhanced ‘liking’ or response reinforcement. J Neurosci 20: 8122–8130.
Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R (2005). Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods 143: 27–32.
Acknowledgements
This work was supported in part by the NIMH MH66216 (XZ), NIMH MH068542 (RH) and NIDA T32 DA07255-13 (ACS). We thank Barbara Cagniard and Jeff Beeler for helping to set up various behavioral paradigms and Jeff Beeler for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanders, A., Hussain, A., Hen, R. et al. Chronic Blockade or Constitutive Deletion of the Serotonin Transporter Reduces Operant Responding for Food Reward. Neuropsychopharmacol 32, 2321–2329 (2007). https://doi.org/10.1038/sj.npp.1301368
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301368
Keywords
This article is cited by
-
5-HT2C receptor blockade reverses SSRI-associated basal ganglia dysfunction and potentiates therapeutic efficacy
Molecular Psychiatry (2020)
-
Dorsal raphe serotonin neurons inhibit operant responding for reward via inputs to the ventral tegmental area but not the nucleus accumbens: evidence from studies combining optogenetic stimulation and serotonin reuptake inhibition
Neuropsychopharmacology (2019)
-
Serotonin transporter gene promoter methylation status correlates with in vivo prefrontal 5-HTT availability and reward function in human obesity
Translational Psychiatry (2017)
-
Not All Antidepressants Are Created Equal: Differential Effects of Monoamine Uptake Inhibitors on Effort-Related Choice Behavior
Neuropsychopharmacology (2016)
-
Decreased Incentive Motivation Following Knockout or Acute Blockade of the Serotonin Transporter: Role of the 5-HT2C Receptor
Neuropsychopharmacology (2016)