Abstract
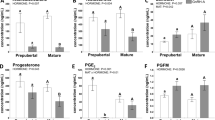
Corticotropin-releasing hormone (CRH) gene and protein expression were examined in the paraventricular nucleus (PVN) of ovariectomized female macaques treated with placebo or hormone therapy (HT) consisting of either estrogen (E) for 28 days, or progesterone (P) for the last 14 of 28 days, or E for 28 days supplemented with P for the last 14 of 28 days using Silastic capsules implanted s.c. in the periscapular region (n=4/group). Perfusion fixed sections (25 μm) at five levels of the PVN (rostral to caudal at 250 μm intervals) were immunostained (ICC) with an antibody to human CRH or processed in an in situ hybridization (ISH) assay with a monkey specific CRH riboprobe. The immunostained CRH-positive area was quantified with a Marianas Stereology Workstation and Slidebook 4.2. There was a significant decrease in the immunological CRH signal with E, P, and E+P treatment as measured by total or average pixels and microns (analysis of variance (ANOVA), p<0.002; Student–Newman–Keul's post hoc test versus placebo control group, p<0.05). There was also a decrease in the number of detectable CRH neurons (ANOVA, p<0.03) with HT. The sections processed for ISH were exposed to autoradiographic films. The CRH mRNA signal was analyzed with NIH Image. The average optical density and positive pixel area of the CRH mRNA signal was significantly suppressed by ovarian HT (ANOVA p<0.002; Student–Newman–Keul's post hoc test versus placebo control group, p<0.05). In summary, 1 month of stable treatment with a moderate dose of E, P or E+P significantly reduced CRH mRNA and protein in the PVN of ovariectomized monkeys. These results suggest that this hormone treatment regimen may increase stress resilience in surgically menopausal primates.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN (2005). Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain 128: 1301–1313.
Barbaccia ML, Serra M, Purdy RH, Biggio G (2001). Stress and neuroactive steroids. Int Rev Neurobiol 46: 243–272.
Bethea CL (1993). Colocalization of progestin receptors with serotonin in raphe neurons of macaque. Neuroendocrinology 57: 1–6.
Bethea CL (1994). Regulation of progestin receptors in raphe neurons of steroid-treated monkeys. Neuroendocrinology 60: 50–61.
Bethea CL, Brown NA, Kohama SG (1996). Steroid regulation of estrogen and progestin receptor messenger ribonucleic acid in monkey hypothalamus and pituitary. Endocrinology 137: 4372–4383.
Bethea CL, Mirkes SJ, Shively CA, Adams MR (2000). Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biol Psychiatry 47: 562–576.
Bethea CL, Lu NZ, Gundlah C, Streicher JM (2002). Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 23: 41–100.
Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL (2005a). Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril 83: 148–155.
Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL (2005b). Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience 132: 151–166.
Carrasco GA, Barker SA, Zhang Y, Damjanoska KJ, Sullivan NR, Garcia F et al (2004). Estrogen treatment increases the levels of regulator of G protein signaling-Z1 in the hypothalamic paraventricular nucleus: possible role in desensitization of 5-hydroxytryptamine1A receptors. Neuroscience 127: 261–267.
Centeno ML, Reddy AP, Smith LJ, Sanchez RL, Henderson JA, Salli NC et al (2007). Serotonin in microdialysate from the mediobasal hypothalamus increases after progesterone administration to estrogen primed macaques. Eur J Pharmacol 555: 67–75.
Dayas CV, Xu Y, Buller KM, Day TA (2000). Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. J Neuroendocrinol 12: 784–794.
de Kloet ER, Joels M, Holsboer F (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6: 463–475.
Dunn AJ, Berridge CW (1990). Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15: 71–100.
Fuller RW (1992). The involvement of serotonin in regulation of pituitary–adrenocortical function. Front Neuroendocrinol 13: 250–270.
Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL (2001). Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry 49: 788–797.
Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL (2000). Distribution of estrogen receptor beta (ERβ) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Mol Brain Res 76: 191–204.
Gundlah C, Lu NZ, Mirkes SJ, Bethea CL (2001). Estrogen receptor beta (ERβ) mRNA and protein in serotonin neurons of macaques. Mol Brain Res 91: 14–22.
Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL (1999). Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Mol Brain Res 63: 325–339.
Hanley NR, Van de Kar LD (2003). Serotonin and the neuroendocrine regulation of the hypothalamic--pituitary-adrenal axis in health and disease. Vitam Horm 66: 189–255.
Himmerich H, Binder EB, Kunzel HE, Schuld A, Lucae S, Uhr M et al (2006). Successful antidepressant therapy restores the disturbed interplay between TNF-alpha system and HPA axis. Biol Psychiatry 60: 882–888.
Holsboer F (1999). The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res 33: 181–214.
Keck ME, Holsboer F (2001). Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides 22: 835–844.
Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS (2006). Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol Psychol 71: 2–11.
Kloet ER (1995). Steroids, stability and stress. Front Neuroendocrinol 16: 416–425.
Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C (1999). Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology 70: 422–430.
Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S (1998). Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 36: 357–378.
Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, O’Byrne KT (2003). The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. J Neuroendocrinol 15: 468–476.
Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M et al (1995). Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept 59: 229–239.
Lu NZ, Bethea CL (2002). Ovarian steroid regulation of 5HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology 27: 12–24.
Luiten PG, ter Horst GJ, Karst H, Steffens AB (1985). The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res 329: 374–378.
Lund TD, Munson DJ, Haldy ME, Handa RJ (2004). Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 16: 272–278.
Matthews KA, Berga SL, Owens JF, Flory JD (1998). Effects of short-term suppression of ovarian hormones on cardiovascular and neuroendocrine reactivity to stress in women. Psychoneuroendocrinology 23: 307–322.
Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM (2004). Estrogen receptor (ER)beta isoforms rather than ERalpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J Neurosci 24: 10628–10635.
Mirkes SJ, Bethea CL (2001). Oestrogen, progesterone and serotonin converge on GABAergic neurones in the monkey hypothalamus. J Neuroendocrinol 13: 182–192.
Moncek F, Duncko R, Jezova D (2003). Repeated citalopram treatment but not stress exposure attenuates hypothalamic-pituitary-adrenocortical axis response to acute citalopram injection. Life Sci 72: 1353–1365.
Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N (1993). The central role of corticotropin-releasing factor (CRF-41) in psychological stress in rats. J Physiol 460: 221–229.
Nemeroff CC (2004). Early-life adversity, CRF dysregulation, and vulnerability to mood and anxiety disorders. Psychopharmacol Bull 38: 14–20.
Owens MJ, Nemeroff CB (1994). Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 40: 288–295.
Patchev VK, Hassan AH, Holsboer DF, Almeida OF (1996). The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 15: 533–540.
Petrov T, Krukoff TL, Jhamandas JH (1992). The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J Comp Neurol 318: 18–26.
Petrov T, Krukoff TL, Jhamandas JH (1994). Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res 277: 289–295.
Portillo F, Carrasco M, Vallo JJ (1998). Separate populations of neurons within the paraventricular hypothalamic nucleus of the rat project to vagal and thoracic autonomic preganglionic levels and express c-Fos protein induced by lithium chloride. J Chem Neuroanat 14: 95–102.
Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF (1994). Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 60: 436–444.
Roy BN, Reid RL, Van Vugt DA (1999). The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology 140: 2191–2198.
Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL (2005). A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res 135: 194–203.
Schule C, Baghai TC, Eser D, Zwanzger P, Jordan M, Buechs R et al (2006). Time course of hypothalamic–pituitary–adrenocortical axis activity during treatment with reboxetine and mirtazapine in depressed patients. Psychopharmacology (Berl) 186: 601–611.
Stout SC, Owens MJ, Nemeroff CB (2002). Regulation of corticotropin-releasing factor neuronal systems and hypothalamic-pituitary-adrenal axis activity by stress and chronic antidepressant treatment. J Pharmacol Exp Ther 300: 1085–1092.
Swanson LW, Sawchenko PE (1983). Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6: 269–324.
Toufexis DJ, Davis C, Hammond A, Davis M (2004). Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J Neurosci 24: 10280–10287.
Vale W, Spiess J, Rivier C, Rivier J (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213: 1394–1397.
Vamvakopoulos NC, Chrousos GP (1993). Structural organization of the 5’ flanking region of the human corticotropin releasing hormone gene. DNA Seq 4: 197–206.
Van de Kar L (1991). Neuroendocrine pharmacology of serotonergic (5HT) neurons. Anim Rev Pharmacol Toxicol 31: 289–320.
Vural P, Akgul C, Canbaz M (2006). Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol Res 54: 298–302.
Xia-Zhang L, Xiao E, Ferin M (1995). A 5-day estradiol therapy, in amounts reproducing concentrations of the early-mid follicular phase, prevents the activation of the hypothalamo-pituitary-adrenal axis by interleukin-1 alpha in the ovariectomized rhesus monkey. J Neuroendocrinol 7: 387–392.
Acknowledgements
Portions of this study were presented at the 36th annual meeting of the Society for Neuroscience, 14–18, October 2006, Altanta, GA and at the 45th annual meeting of the American College of Neuropsychopharmacology, 3–7, December 2006, Hollywood, FL. We thank Dr Wylie Vale for his generous gift of antiserum to human CRH.
Supported by NIH grants MH62677 to CLB, U54 contraceptive Center Grant HD 18185, and RR000163 for the operation of ONPRC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure
The authors have no financial interests to disclose.
Rights and permissions
About this article
Cite this article
Bethea, C., Centeno, M. Ovarian Steroid Treatment Decreases Corticotropin-Releasing Hormone (CRH) mRNA and Protein in the Hypothalamic Paraventricular Nucleus of Ovariectomized Monkeys. Neuropsychopharmacol 33, 546–556 (2008). https://doi.org/10.1038/sj.npp.1301442
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301442