Abstract
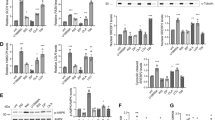
To measure the long-term changes in weight and plasma lipids after switching antipsychotic treatment to ziprasidone, three 52-week, open-label extension studies of ziprasidone in outpatients (N=185) with schizophrenia or schizoaffective disorder successfully completing one of three, 6-week switch studies were carried out. Pre-switch treatment consisted of risperidone (n=43), olanzapine (n=71), or conventional antipsychotic agents (n=71). The maximum length of exposure to ziprasidone was 58 weeks. Nonfasting total cholesterol and triglyceride levels were obtained at baseline and at weeks 6, 19, 32, 45, and 58. Weight was measured at baseline and during each follow-up visit; height was recorded at baseline for the purpose of body mass index (BMI) calculation. Efficacy measures included the Positive and Negative Syndrome Scale and Clinical Global Impression—Severity scale which were obtained at baseline and major follow-up points. Clinically significant sustained improvements in weight, BMI, total cholesterol, and triglyceride levels were observed among patients switched to ziprasidone from risperidone or olanzapine. Switching from conventional antipsychotics was not associated with significant changes in weight and lipid parameters. Mean reductions in weight from baseline to study endpoint were 9.8 kg (p<0.001) and 6.9 kg (p<0.005) for patients previously treated with olanzapine and risperidone, respectively. These findings demonstrate that switching from risperidone or olanzapine to ziprasidone is associated with sustained, clinically significant improvements in weight and plasma lipids.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC et al (1999). Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 156: 1686–1696.
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity (2004). Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27: 596–601.
Arato M, O'Connor R, Meltzer HY (2002). A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol 17: 207–215.
Baptista T, Martinez J, Lacruz A, Rangel N, Beaulieu S, Serrano A et al (2006). Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry 51: 192–196.
Baxter DN (1996). The mortality experience of individuals on the Salford psychiatric case register. I. All-cause mortality. Br J Psychiatry 168: 772–779.
Bazett HC (1920). An analysis of the time-relations of electrocardiograms. Heart 7: 353–370.
Bobes J, Rejas J, Garcia-Garcia M, Rico-Villademoros F, Garcia-Portilla MP, Fernandez I et al (2003). Weight gain in patients with schizophrenia treated with risperidone, olanzapine, quetiapine or haloperidol: results of the EIRE study. Schizophr Res 62: 77–88.
Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R (2005). Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 66: 205–212.
Brown S, Birtwistle J, Roe L, Thompson C (1999). The unhealthy lifestyle of people with schizophrenia. Psychol Med 29: 697–701.
Brown S, Inskip H, Barraclough B (2000). Causes of the excess mortality of schizophrenia. Br J Psychiatry 177: 212–217.
Casey DE, Carson WH, Saha AR, Liebeskind A, Ali MW, Jody D et al (2003). Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology (Berl) 166: 391–399.
Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M et al (2004). Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry 65 (Suppl 7): 4–18.
Casey DE, Zorn SH (2001). The pharmacology of weight gain with antipsychotics. J Clin Psychiatry 62 (Suppl 7): 4–10.
Coodin S (2001). Body mass index in persons with schizophrenia. Can J Psychiatry 46: 549–555.
Craig SR, Amin RV, Russell DW, Paradise NF (2000). Blood cholesterol screening influence of fasting state on cholesterol results and management decisions. J Gen Intern Med 15: 395–399.
Daumit GL, Goldberg RW, Anthony C, Dickerson F, Brown CH, Kreyenbuhl J et al (2005). Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis 193: 641–646.
Desmeules S, Arcand-Bosse JF, Bergeron J, Douville P, Agharazi M (2005). Nonfasting non-high-density lipoprotein cholesterol is adequate for lipid management in hemodialysis patients. Am J Kidney Dis 45: 1067–1072.
Eberly LE, Stamler J, Neaton JD (2003). Mortality after 16 years for participants randomized to the Multiple Risk Factor Intervention Trial. Arch Intern Med 163: 1077–1083.
Eyre H, Kahn R, Robertson RM (2004). Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Diabetes Care 27: 1812–1824.
Farwell WR, Stump TE, Wang J, Tafesse E, L'Italien G, Tierney WM (2004). Weight gain and new onset diabetes associated with olanzapine and risperidone. J Gen Intern Med 19: 1200–1205.
Faulkner G, Cohn T, Remington G (2007). Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev 1: CD005148.
Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshminarayanan M, Casey DE et al (2001). Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 101: 277–288.
Goldman LS (1999). Medical illness in patients with schizophrenia. J Clin Psychiatry 60 (Suppl 21): 10–15.
Hebert PR, Gaziano JM, Chan KS, Hennekens CH (1997). Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 278: 313–321.
Henderson DC, Cagliero E, Copeland PM, Borba CP, Evins E, Hayden D et al (2006). Dietary intake profile of patients with schizophrenia. Ann Clin Psychiatry 18: 99–105.
Henderson DC, Copeland PM, Daley TB, Borba CP, Cather C, Nguyen DD et al (2005). A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. Am J Psychiatry 162: 954–962.
Henderson DC, Fan X, Copeland PM, Borba CP, Daley TB, Nguyen DD et al (2007). A double-blind, placebo-controlled trial of sibutramine for clozapine-associated weight gain. Acta Psychiatr Scand 115: 101–105.
Hennekens CH (1998). Increasing burden of cardiovascular disease: current knowledge and future directions for research on risk factors. Circulation 97: 1095–1102.
Jin H, Meyer JM, Jeste DV (2004). Atypical antipsychotics and glucose dysregulation: a systematic review. Schizophr Res 71: 195–212.
Joyce AT, Harrison DJ, Loebel AD, Carter CT, Ollendorf DA (2006). Effect of initial ziprasidone dose on length of therapy in schizophrenia. Schizophr Res 83: 285–292.
Koro CE, Fedder DO, L'Italien GJ, Weiss S, Magder LS, Kreyenbuhl J et al (2002a). An assessment of the independent effects of olanzapine and risperidone exposure on the risk of hyperlipidemia in schizophrenic patients. Arch Gen Psychiatry 59: 1021–1026.
Koro CE, Fedder DO, L'Italien GJ, Weiss SS, Magder LS, Kreyenbuhl J et al (2002b). Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 325: 243.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO et al (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353: 1209–1223.
Littrell KH, Hilligoss NM, Kirshner CD, Petty RG, Johnson CG (2003). The effects of an educational intervention on antipsychotic-induced weight gain. J Nurs Scholarsh 35: 237–241.
Mamo D, Kapur S, Shammi CM, Papatheodorou G, Mann S, Therrien F et al (2004). A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry 161: 818–825.
McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L et al (2005). Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 80: 19–32.
McIntyre RS, Trakas K, Lin D, Balshaw R, Hwang P, Robinson K et al (2003). Risk of weight gain associated with antipsychotic treatment: results from the Canadian National Outcomes Measurement Study in schizophrenia. Can J Psychiatry 48: 689–694.
McMahon FG, Fujioka K, Singh BN, Mendel CM, Rowe E, Rolston K et al (2000). Efficacy and safety of sibutramine in obese white and African American patients with hypertension: a 1-year, double-blind, placebo-controlled, multicenter trial. Arch Intern Med 160: 2185–2191.
Menza M, Vreeland B, Minsky S, Gara M, Radler DR, Sakowitz M (2004). Managing atypical antipsychotic-associated weight gain: 12-month data on a multimodal weight control program. J Clin Psychiatry 65: 471–477.
Meyer JM, Koro CE (2004). The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res 70: 1–17.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2001). Executive summary of the third report of the National Cholesterol Education Program (NCEP). JAMA 285: 2486–2497.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002). Third report of the National Cholesterol Education Program (NCEP)—final report. Circulation 106: 3143–3421.
National Heart, Lung and Blood Institute (1998). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—the Evidence Report. National Institutes of Health: Bethesda, MD.
Nemeroff CB (1997). Dosing the antipsychotic medication olanzapine. J Clin Psychiatry 58 (Suppl 10): 45–49.
Nemeroff CB, Lieberman JA, Weiden PJ, Harvey PD, Newcomer JW, Schatzberg AF et al (2005). From clinical research to clinical practice: a 4-year review of ziprasidone. CNS Spectr 10: S1–S20.
Newcomer JW (2005). Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19 (Suppl 1): 1–93.
Newcomer JW, Haupt DW (2006). The metabolic effects of antipsychotic medications. Can J Psychiatry 51: 480–491.
Newcomer JW, Haupt DW, Fucetola R, Melson AK, Schweiger JA, Cooper BP et al (2002). Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry 59: 337–345.
Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Serdula M et al (2004). Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med 117: 762–774.
Padwal R, Li SK, Lau DC (2004). Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev 3: CD004094.
Ryan MC, Collins P, Thakore JH (2003). Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry 160: 284–289.
Sheppard L, Kristal AR, Kushi LH (1991). Weight loss in women participating in a randomized trial of low-fat diets. Am J Clin Nutr 54: 821–828.
Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA et al (2005). Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 163: 611–622.
Weiden PJ, Daniel DG, Simpson G, Romano SJ (2003a). Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J Clin Psychopharmacol 23: 595–600.
Weiden P, Simpson G, Potkin S, O'Sullivan RL (2003b). Switching to ziprasidone from conventional antipsychotics, olanzapine, or risperidone in stable outpatients with schizophrenia. J Clin Psychiatry 64: 580–588.
Weiss R, Harder M, Rowe J (2003). The relationship between nonfasting and fasting lipid measurements in patients with or without type 2 diabetes mellitus receiving treatment with 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Clin Ther 25: 1490–1497.
Wirshing DA (2004). Schizophrenia and obesity: impact of antipsychotic medications. J Clin Psychiatry 65 (Suppl 18): 13–26.
Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J et al (1999). Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 60: 358–363.
Acknowledgements
Editorial support was provided by Frances Brentson at PAREXEL and funding was provided by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosures
Dr Lebovitz is a consultant to Amylin Pharmaceuticals Inc.; a member of the Speaker Bureau for Amylin Pharmaceuticals Inc., GlaxoSmithKline, Sanofi Aventis Pharmaceuticals Inc., Bayer Corporation; stock or investment holder: Amylin Pharmaceuticals Inc., GlaxoSmithKline, Bayer Corporation, and Bristol-Myers Squibb Company.
Dr Loebel is an employee of Pfizer Inc.
Dr Newcomer has research grants from The National Institute of Mental Health, The National Alliance for Research on Schizophrenia and Depression, Sidney R Baer Jr Foundation, Janssen, Bristol-Myers Squibb, and Pfizer Inc., and is a consultant to AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Pfizer Inc., Organon, Solvay, and Wyeth.
Dr Weiden has research grants from AstraZeneca, Bristol-Myers Squibb, Janssen, and Pfizer, and is a consultant to AstraZeneca, Bristol-Myers Squibb, Janssen, Pfizer Inc., Shire, and Vanda Pharmaceuticals.
Dr Yang is an employee of Pfizer Inc.
Rights and permissions
About this article
Cite this article
Weiden, P., Newcomer, J., Loebel, A. et al. Long-Term Changes in Weight and Plasma Lipids during Maintenance Treatment with Ziprasidone. Neuropsychopharmacol 33, 985–994 (2008). https://doi.org/10.1038/sj.npp.1301482
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301482
Keywords
This article is cited by
-
Weight changes before and after lurasidone treatment: a real-world analysis using electronic health records
Annals of General Psychiatry (2017)
-
Current Data on and Clinical Insights into the Treatment of First Episode Nonaffective Psychosis: A Comprehensive Review
Neurology and Therapy (2016)
-
Amélioration de la santé cardiovasculaire par l’exercice physique chez les individus atteints de schizophrénie : un guide de pratique
Obésité (2015)
-
Evidence review and clinical guidance for the use of ziprasidone in Canada
Annals of General Psychiatry (2013)
-
The Efficacy and Safety of Switching to Ziprasidone from Olanzapine in Patients with Bipolar I Disorder: An 8-Week, Multicenter, Open-Label Study
Clinical Drug Investigation (2013)