Abstract
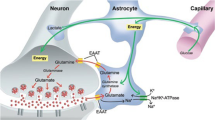
Altered glutamate signaling contributes to a myriad of neural disorders, including schizophrenia. While synaptic levels are intensely studied, nonvesicular release mechanisms, including cystine–glutamate exchange, maintain high steady-state glutamate levels in the extrasynaptic space. The existence of extrasynaptic receptors, including metabotropic group II glutamate receptors (mGluR), pose nonvesicular release mechanisms as unrecognized targets capable of contributing to pathological glutamate signaling. We tested the hypothesis that activation of cystine–glutamate antiporters using the cysteine prodrug N-acetylcysteine would blunt psychotomimetic effects in the rodent phencyclidine (PCP) model of schizophrenia. First, we demonstrate that PCP elevates extracellular glutamate in the prefrontal cortex, an effect that is blocked by N-acetylcysteine pretreatment. To determine the relevance of the above finding, we assessed social interaction and found that N-acetylcysteine reverses social withdrawal produced by repeated PCP. In a separate paradigm, acute PCP resulted in working memory deficits assessed using a discrete trial t-maze task, and this effect was also reversed by N-acetylcysteine pretreatment. The capacity of N-acetylcysteine to restore working memory was blocked by infusion of the cystine–glutamate antiporter inhibitor (S)-4-carboxyphenylglycine into the prefrontal cortex or systemic administration of the group II mGluR antagonist LY341495 indicating that the effects of N-acetylcysteine requires cystine–glutamate exchange and group II mGluR activation. Finally, protein levels from postmortem tissue obtained from schizophrenic patients revealed significant changes in the level of xCT, the active subunit for cystine–glutamate exchange, in the dorsolateral prefrontal cortex. These data advance cystine–glutamate antiporters as novel targets capable of reversing the psychotomimetic effects of PCP.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abbott WA, Meister A (1986). Intrahepatic transport and utilization of biliary glutathione and its metabolites. Proc Natl Acad Sci USA 83: 1246–1250.
Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y et al (2006). Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci 9: 119–126.
Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE (2007). Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J Neurosci 27: 111–123.
Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S et al (2003). Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6: 743–749.
Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW (2002). The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22: 9134–9141.
Bannai S (1984). Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. J Biol Chem 259: 2435–2440.
Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald III A, Noll DC et al (2001). Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry 58: 280–288.
Baskys A, Malenka RC (1991). Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol 444: 687–701.
Bradford HF, Young AM, Crowder JM (1987). Continuous glutamate leakage from brain cells is balanced by compensatory high-affinity reuptake transport. Neurosci Lett 81: 296–302.
Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A et al (1994). Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry 151: 20–26.
Bunney WE, Bunney BG (2000). Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev 31: 138–146.
Cabungcal JH, Nicolas D, Kraftsik R, Cuenod M, Do KQ, Hornung JP (2006). Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: relevance to schizophrenia. Neurobiol Dis 22: 624–637.
Cartmell J, Schoepp DD (2000). Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75: 889–907.
Chaki S, Yoshikawa R, Okuyama S (2006). Group II metabotropic glutamate receptor-mediated regulation of dopamine release from slices of rat nucleus accumbens. Neurosci Lett 404: 182–186.
Chavez-Noriega LE, Schaffhauser H, Campbell UC (2002). Metabotropic glutamate receptors: potential drug targets for the treatment of schizophrenia. Curr Drug Target CNS Neurol Disord 1: 261–281.
Cochilla AJ, Alford S (1998). Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron 20: 1007–1016.
Corbett R, Camacho F, Woods AT, Kerman LL, Fishkin RJ, Brooks K et al (1995). Antipsychotic agents antagonize non-competitive N-methyl-D-aspartate antagonist-induced behaviors. Psychopharmacology (Berl) 120: 67–74.
D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP et al (2007). mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA 104: 1995–2000.
Deneke SM, Fanburg BL (1989). Regulation of cellular glutathione. Am J Physiol 257: L163–L173.
Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D et al (2000). Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci 12: 3721–3728.
Faltus F, Hynek K, Dolezalova V, Kumnickova Z, Zemek P (1973). Experience in the treatment of schizophrenia with clozapine. Act Nerv Super (Praha) 15: 95.
Featherstone DE, Rushton E, Broadie K (2002). Developmental regulation of glutamate receptor field size by nonvesicular glutamate release. Nat Neurosci 5: 141–146.
Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH (2005). Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 57: 123–131.
Harvey PD, McClure MM (2006). Pharmacological approaches to the management of cognitive dysfunction in schizophrenia. Drugs 66: 1465–1473.
Hauber W (1993). Clozapine improves dizocilpine-induced delayed alteration impairment in rats. J Neural Transm Gen Sect 94: 223–233.
Herrera-Marschitz M, You ZB, Goiny M, Meana JJ, Silveira R, Godukhin OV et al (1996). On the origin of extracellular glutamate levels monitored in the basal ganglia of the rat by in vivo microdialysis. J Neurochem 66: 1726–1735.
Homayoun H, Jackson ME, Moghaddam B (2005). Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol 93: 1989–2001.
Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW (1999a). The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther 289: 412–416.
Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW (1999b). The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther 289: 412–416.
Huang Y, Dai Z, Barbacioru C, Sadee W (2005). Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res 65: 7446–7454.
Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U (1999). Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci USA 96: 8733–8738.
Jackson ME, Homayoun H, Moghaddam B (2004). NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA 101: 8467–8472.
Javitt DC, Zukin SR (1991). Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148: 1301–1308.
Kilbride J, Huang LQ, Rowan MJ, Anwyl R (1998). Presynaptic inhibitory action of the group II metabotropic glutamate receptor agonists, LY354740 and DCG-IV. Eur J Pharmacol 356: 149–157.
Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, Kim DK et al (2001). Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim Biophys Acta 1512: 335–344.
Kohr G, Eckardt S, Luddens H, Monyer H, Seeburg PH (1994). NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron 12: 1031–1040.
Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003). NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 169: 215–233.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO et al (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353: 1209–1223.
Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA (2003). Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117: 697–706.
Lu L, Hope BT, Shaham Y (2004). The cystine-glutamate transporter in the accumbens: a novel role in cocaine relapse. Trends Neurosci 27: 74–76.
Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R (1959). Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry 81: 363–369.
MacDonald III AW, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ et al (2005). Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry 162: 475–484.
Malhotra AK, Adler CM, Kennison SD, Elman I, Pickar D, Breier A (1997). Clozapine blunts N-methyl-D-aspartate antagonist-induced psychosis: a study with ketamine. Biol Psychiatry 42: 664–668.
Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L et al (2005). A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci 25: 4755–4765.
Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000). Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292: 76–87.
Meister A (1985). Methods for the selective modification of glutathione metabolism and study of glutathione transport. Methods Enzymol 113: 571–585.
Meister A (1991). Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther 51: 155–194.
Moghaddam B, Adams B, Verma A, Daly D (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17: 2921–2927.
Moghaddam B, Adams BW (1998). Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281: 1349–1352.
Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK (2005). Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 25: 6389–6393.
Paxinos G, Watson C (1986). The Rat Brain in Stereotaxic Coordinates. Academic Press: New York.
Pearlson GD (1981). Psychiatric and medical syndromes associated with phencyclidine (PCP) abuse. Johns Hopkins Med J 148: 25–33.
Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A (1997). Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron 19: 1237–1248.
Pileblad E, Magnusson T (1992). Increase in rat brain glutathione following intracerebroventricular administration of gamma-glutamylcysteine. Biochem Pharmacol 44: 895–903.
Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL (1998). Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Arch Gen Psychiatry 55: 205–211.
Sams-Dodd F (1999). Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Rev Neurosci 10: 59–90.
Sato H, Tamba M, Ishii T, Bannai S (1999). Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274: 11455–11458.
Schoepp DD (2001). Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299: 12–20.
Schoepp DD, Jane DE, Monn JA (1999). Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 38: 1431–1476.
Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH (2006). Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci 26: 10514–10523.
Sies H (1999). Glutathione and its role in cellular functions. Free Radic Biol Med 27: 916–921.
Sigrist SJ, Thiel PR, Reiff DF, Lachance PE, Lasko P, Schuster CM (2000). Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature 405: 1062–1065.
Steullet P, Neijt HC, Cuenod M, Do KQ (2006). Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience 137: 807–819.
Takahata R, Moghaddam B (2003). Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology 28: 1117–1124.
Timmerman W, Westerink BH (1997). Brain microdialysis of GABA and glutamate: what does it signify? Synapse 27: 242–261.
Uylings HB, Groenewegen HJ, Kolb B (2003). Do rats have a prefrontal cortex? Behav Brain Res 146: 3–17.
Valenti O, Conn PJ, Marino MJ (2002). Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J Cell Physiol 191: 125–137.
Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H et al (2000). Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287: 864–869.
Weinberger DR (1987). Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44: 660–669.
Williamson JM, Meister A (1981). Stimulation of hepatic glutathione formation by administration of L-2-oxothiazolidine-4-carboxylate, a 5-oxo-L-prolinase substrate. Proc Natl Acad Sci USA 78: 936–939.
Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW (2002a). Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300: 162–171.
Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW (2002b). Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther 303: 608–615.
Acknowledgements
MH71672 (DAB), MH53327 (JMW), MH-45212 (VH), MH64673 (VH), and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (DAB). We thank Hideyo Sato for donating mxCT cDNA.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
Dr James Meador-Woodruff is the editor in chief of Neuropsychopharmacology and the ACNP website and receives a stipend from ACNP for these services. The remaining authors do not have a conflict of interest or any relationship with outside organizations to disclose.
Rights and permissions
About this article
Cite this article
Baker, D., Madayag, A., Kristiansen, L. et al. Contribution of Cystine–Glutamate Antiporters to the Psychotomimetic Effects of Phencyclidine. Neuropsychopharmacol 33, 1760–1772 (2008). https://doi.org/10.1038/sj.npp.1301532
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301532
Keywords
This article is cited by
-
Exploring the mRNA expression level of RELN in peripheral blood of schizophrenia patients before and after antipsychotic treatment
Hereditas (2020)
-
Effects of N-acetylcysteine on brain glutamate levels and resting perfusion in schizophrenia
Psychopharmacology (2018)
-
Implication of the glutamate–cystine antiporter xCT in schizophrenia cases linked to impaired GSH synthesis
npj Schizophrenia (2017)
-
Global quantitative analysis of phosphorylation underlying phencyclidine signaling and sensorimotor gating in the prefrontal cortex
Molecular Psychiatry (2016)
-
Imaging glutamate in schizophrenia: review of findings and implications for drug discovery
Molecular Psychiatry (2014)