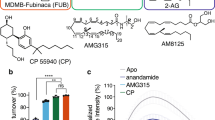
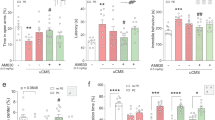
Abstract
Cannabinoid CB1 receptors are densely expressed on striatal projection neurons expressing dopamine D1 or D2 receptors. However, the specific neuronal distribution of CB1 receptors within the striatum is not known. Previous research has established that the endocannabinoid system controls facilitation of behavior by dopamine D2 receptors, but it is not clear if endocannabinoids also modulate D1 receptor-mediated motor behavior. In the present study, we show that cannabinoid CB1 receptor mRNA is present in striatonigral neurons expressing substance P and dopamine D1 receptors, as well as in striatopallidal neurons expressing enkephalin and dopamine D2 receptors. We explored the functional relevance of the interaction between dopamine D1 and D2 receptors and cannabinoid CB1 receptors with behavioral pharmacology experiments. Potentiation of endogenous cannabinoid signaling by the uptake blocker AM404 blocked dopamine D1 receptor-mediated grooming and D2 receptor-mediated oral stereotypies. In addition, contralateral turning induced by unilateral intrastriatal infusion of D1 receptor agonists is counteracted by AM404 and potentiated by the cannabinoid antagonist SR141716A. These results indicate that the endocannabinoid system negatively modulates D1 receptor-mediated behaviors in addition to its previously described effect on dopamine D2 receptor-mediated behaviors. The effect of AM404 on grooming behavior was absent in dopamine D1 receptor knockout mice, demonstrating its dependence on D1 receptors. This study indicates that the endocannabinoid system is a relevant negative modulator of both dopamine D1 and D2 receptor-mediated behaviors, a finding that may contribute to our understanding of basal ganglia motor disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aldridge JW, Berridge KC (1998). Coding of serial order by neostriatal neurons: a ‘natural action’ approach to movement sequence. J Neurosci 18: 2777–2787.
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW et al (2000). Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404: 84–87.
Beltramo M, Rodriguez de Fonseca F, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G et al (2000). Reversal of dopamine D2 receptor responses by an anandamide transport inhibitor. J. Neurosci 20: 3401–3407.
Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D (1997). Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 277: 1094–1097.
Berretta S, Sachs Z, Graybiel AM (1999). Cortically driven Fos induction in the striatum is amplified by local dopamine D2-class receptor blockade. Eur J Neurosci 11: 4309–4319.
Bisogno T, MacCarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agor A, Hillard C et al (2001). The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur J Biochem 268: 1982–1989.
Bolam JP, Hanley JJ, Booth PA, Bevan MD (2000). Synaptic organization of the basal ganglia. J Anat 196(Part 4): 527–542.
Cadogan AK, Alexander SP, Boyd EA, Kendall DA (1997). Influence of cannabinoids on electrically evoked dopamine release and cyclic AMP generation in the rat striatum. J Neurochem 69: 1131–1137.
Davidkova G, Zhou L-W, Morabito M, Zhang S-P, Weiss B (1998). D2 dopamine antisense RNA expression vector, unlike haloperidol, produces long-term inhibition of D2 dopamine-mediated behaviors without causing up-regulation of D2 dopamine receptors. J Pharmacol Exp Ther 285: 1187–1196.
De Lago E, de Miguel R, Lastres-Becker I, Ramos JA, Fernandez-Ruiz J (2004). Involvement of vanilloid-like receptors in the effects of anandamide on motor behavior and nigrostriatal dopaminergic activity: in vivo and in vitro evidence. Brain Res 1007: 152–159.
El Banoua F, Caraballo I, Flores JA, Galan-Rodriguez B, Fernandez-Espejo E (2004). Effects on turning of microinjections into basal ganglia of D1 and D2 dopamine receptors agonists, and the cannabinoid CB1 antagonist SR141716A in a rat Parkinson's model. Neurobiol Dis 16: 377–385.
Fernandez-Espejo E, Caraballo I, Rodriguez de Fonseca F, El Banoua F, Ferrer B, Flores JA et al (2005). Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with very severe nigral lesion in experimental Parkinsonism. Neurobiol Dis 18: 591–601.
Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A (2003). Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci 18: 1607–1614.
French ED, Dillon K, Wu X (1997). Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 3: 649–652.
Gerdeman G, Lovinger DM (2001). CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol 85: 468–471.
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma Jr FJ et al (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250: 1429–1432.
Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D (1999). Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2: 358–363.
Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG (2003). Evidence against the presence of an anandamide transporter. Proc Nat Acad Sci USA 100: 4269–4274.
Glass M, Felder CC (1997). Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci 17: 5327–5333.
Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D et al (2002). Experimental Parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci 22: 6900–6907.
Hauber W (1998). Involvement of basal ganglia transmitter systems in movement initiation. Prog Neurobiol 56: 507–540.
Herkenham M, Lynn AB, De Costa BR, Richfield EK (1991). Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res 547: 267–274.
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR et al (1990). Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87: 1932–1936.
Hermann H, Marsicano G, Lutz B (2002). Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 109: 451–460.
Julián MD, Martín AB, Cuéllar B, Rodríguez de Fonseca F, Navarro M, Moratalla R et al (2003). Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience 119: 309–318.
Kalueff AV, Tuohimaa P (2005). Mouse grooming microstructure is a reliable anxiety marker bidirectionally sensitive to GABAergic drugs. Eur J Pharmacol 508: 147–153.
Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M (2005). Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol 67: 1697–1704.
Keefe KA, Gerfen CR (1995). D1–D2 dopamine receptor synergy in striatum: effects of intrastriatal infusions of dopamine agonists and antagonists on immediate early gene expression. Neuroscience 66: 903–913.
Löschmann PA, Wüllner U, Heneka MT, Schulz JB, Kunow M, Wachtel H et al (1997). Differential interaction of competitive NMDA and AMPA antagonists with selective dopamine D-1 and D-2 agonists in a rat model of Parkinson's disease. Synapse 26: 381–391.
Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ (2004). A single in-vivo exposure to delta 9-THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci 7: 585–586.
McKenzie S, Kemm RE, Wilcock LN (1984). The Basal Ganglia. Structure and Function. Plenum Press: New York.
McPherson RJ, Marshall JF (1996). Intrastriatal AP5 differentially affects behaviors induced by local infusions of D1 vs D2 dopamine agonists. Brain Res 739: 19–25.
Molloy AG, Waddington JL (1987). Assessment of grooming and other behavioural responses to the D-1 dopamine receptor agonist SK & F 38393 and its R- and S-enantiomers in the intact adult rat. Psychopharmacol 92: 164–168.
Molloy AG, Waddington JL (1984). Dopaminergic behavior stereospecifically promoted by the D-1 agonist R-SKF 38393 and selectively blocked by the D-1 antagonist SCH 23390. Psychopharmacology 82: 409–410.
Moratalla R, Xu M, Tonegawa S, Graybiel AM (1996). Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci USA 93: 14928–14933.
Nicola SM, Surmeier DJ, Malenka RC (2000). Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185–215.
O’Connor WT (1998). Functional neuroanatomy of the basal ganglia as studied by dual-probe microdyalisis. Nucl Med Biol 25: 743–746.
Onn SP, West AR, Grace AA (2000). Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci 23(suppl): S48–S56.
Paul ML, Graybiel AM, David JC, Robertson HA (1992). D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson's disease. J Neurosci 12: 3729–3742.
Pavón N, Martín AB, Mendialdua A, Moratalla R (2006). ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry 59: 64–74.
Paxinos G, Watson C (2005). The Rat Brain in Stereotaxic Coordinates. Elsevier: Amsterdam.
Pertwee RG (1999). Pharmacology of cannabinoid receptor ligands. Curr Med Chem 6: 635–664.
Rivera A, Cuellar B, Giron FJ, Grandy DK, de la Calle A, Moratalla R (2002a). Dopamine D4 receptors are heterogeneously distributed in the striosomes/matrix compartments of the striatum. J Neurochem 80: 219–229.
Rivera A, Alberti I, Martin AB, Narvaez JA, de la Calle A, Moratalla R (2002b). Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur J Neurosci 16: 2049–2058.
Rodriguez de Fonseca F, Del Arco I, Martin-Calderon JL, Gorriti MA, Navarro M (1998). Role of the endogenous cannabinoid system in the regulation of motor activity. Neurobiol Dis 5: 483–501.
Rodriguez de Fonseca F, Martin Calderon JL, Mechoulam R, Navarro M (1994). Repeated stimulation of D1 dopamine receptors enhances (−)-11-hydroxy-delta-8-tetrahydrocannabinol-dimethyl-heptyl-induced catalepsy in male rats. Neuroreport 5: 761–765.
Ronesi J, Gerdeman GL, Lovinger DM (2004). Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci 24: 1673–1679.
Routtenberg A (1972). Intracranial chemical injection and behavior: a critical review. Behav Biol 7: 601–641.
Sañudo-Peña MC, Force M, Tsou K, Miller AS, Walker JM (1998a). Effects of intrastriatal cannabinoids on rotational behaviors in rats: interactions with the dopaminergic system. Synapse 30: 221–226.
Sañudo-Peña MC, Patrick SL, Khen S, Patrick RL, Tsou K, Walker JM (1998b). Cannabinoid effects in basal ganglia in a rat model of Parkinson's disease. Neurosci Lett 248: 171–174.
Schwarting RKW, Huston JP (1996). The unilateral 6-hydroxydopamine lesion model in behavioral brain research: analysis of functional deficits, recovery and treatments. Prog Neurobiol 50: 275–331.
Starr BS, Starr MS (1986a). Grooming in the mouse is stimulated by the dopamine D1 agonist SKF 38393 and by low doses of the D1 antagonist SCH 23390, but is inhibited by dopamine D2 agonists, D2 antagonists and high doses of SCH 23390. Pharmacol Biochem Behav 24: 837–839.
Starr BS, Starr MS (1986b). Differential effects of dopamine D1 and D2 agonists and antagonists on velocity of movement, rearing and grooming in the mouse. Implications for the roles of D1 and D2 receptors. Neuropharmacology 25: 455–463.
Svenningsson P, Fredholm BB, Bloch B, Le Moine C (2000). Co-stimulation of D1/D5 and D2 dopamine receptors leads to an increase in c-fos messenger RNA in cholinergic interneurons and a redistribution of c-fos messenger RNA in striatal projection neurons. Neuroscience 98: 749–757.
Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411.
Tzavara ET, Li DL, Moutsimilli L, Bisogno T, Di Marzo V, Phebus LA et al (2006). Endocannabinoids activate transient receptor potential vanilloid 1 receptors to reduce hyperdopaminergia-related hyperactivity: therapeutic implications. Biol Psychiatry 59: 508–515.
Ungerstedt U, Arbuthnott GW (1970). Quantitative recording of rotational behavior in rats after 6-hydroxydopamine lesions of the nigrostriatal dopamine system. Brain Res 24: 486–493.
Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM et al (1994). Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 79: 729–742.
You ZB, Herrera-Marschitz M, Nylander I, Goiny M, O’Connor WT, Ungerstedt U et al (1994). The striatonigral dynorphin pathway of the rat studied with in vivo microdialysis. II. Effects of dopamine D1 and D2 receptor agonists. Neuroscience 63: 427–434.
Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED (2000). The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol 396: 39–42.
Acknowledgements
This work was supported by MEC SAF2003-4864, SAF 2004-07762, C03/06/02/NAC, Delegación del Gobierno para el Plan Nacional Sobre Drogas and FIS (Red temática de Trastornos Adictivos, RD06/0001) CIBERNED to RM, EFE, MN, and FRF; FIS031004 PI 071073 to RM, and FIS040155 and Junta de Andalucia CVI127 to EFE.
Author information
Authors and Affiliations
Corresponding authors
Additional information
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Martín, A., Fernandez-Espejo, E., Ferrer, B. et al. Expression and Function of CB1 Receptor in the Rat Striatum: Localization and Effects on D1 and D2 Dopamine Receptor-Mediated Motor Behaviors. Neuropsychopharmacol 33, 1667–1679 (2008). https://doi.org/10.1038/sj.npp.1301558
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301558
Keywords
This article is cited by
-
Oleoylethanolamide attenuates cocaine-primed reinstatement and alters dopaminergic gene expression in the striatum
Behavioral and Brain Functions (2023)
-
Acute cannabinoids impair association learning via selectively enhancing synaptic transmission in striatonigral neurons
BMC Biology (2022)
-
A novel allosteric modulator of the cannabinoid CB1 receptor ameliorates hyperdopaminergia endophenotypes in rodent models
Neuropsychopharmacology (2021)
-
On the Biomedical Properties of Endocannabinoid Degradation and Reuptake Inhibitors: Pre-clinical and Clinical Evidence
Neurotoxicity Research (2021)
-
Genetic deletion of dopamine D1 receptors increases the sensitivity to cannabinoid CB1 receptor antagonist-precipitated withdrawal when compared with wild-type littermates: studies in female mice repeatedly exposed to the Spice cannabinoid HU-210
Psychopharmacology (2021)