Abstract
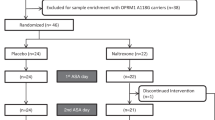
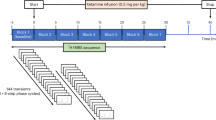
Amphetamine abuse and dependence is a global health concern with a collateral increase in medical and social problems. Although some of the neurobiological mechanisms underlying amphetamine dependence and its devastating effects in humans are known, the development of rational and evidence-based treatment is lagging. There is evidence from preclinical studies suggesting that the endogenous opioid system plays a role in mediating some of the behavioral and neurochemical effects of amphetamine in a variety of controlled settings. In the present study we assessed the effects of naltrexone, an opioid antagonist (50 mg) on the subjective physiological and biochemical response to dexamphetamine (30 mg) in 20 amphetamine-dependent patients. Patients received naltrexone/amphetamine followed by placebo/amphetamine, 1 week apart in a randomized double-blind placebo-controlled design. The primary objective of the study was to evaluate the effect of pretreatment with naltrexone on the subjective response to amphetamine, using a Visual Analog Scale. The secondary objective was to investigate the effects of naltrexone on physiological and biochemical responses to amphetamine, as measured by changes in blood pressure, heart rate, skin conductance, and cortisol. Naltrexone significantly attenuated the subjective effects produced by dexamphetamine in dependent patients (p<0.001). Pretreatment with naltrexone also significantly blocked the craving for dexamphetamine (p<0.001). There was no difference between the groups on the physiological measures. The results suggest that the subjective effects of amphetamine could be modulated via the endogenous opioid system. The potential of naltrexone as an adjunct pharmaceutical for amphetamine dependence is promising.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK (1999). Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry 156: 1758–1764.
Boxenbaum HG, Riegelman S, Elashoff RM (1974). Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm 2: 123–148.
CAN (2001). Drogutvecklingen i Sverige, rapport 99. Rapport nr10 (1999). Folkhälsoinstitutet: Centralförbundet för Alkol och Narkotikaupplysning, Stockholm [The development of drug use in Sweden, report 2001].
Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R et al (2000). A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol 35: 587–593.
Cushman P Jr, Kreek MJ (1974). Methadone-maintained patients. Effect of methadone on plasma testosterone, FSH, LH, and prolactin. NY State J Med 74: 1970–1973.
Dunne A (1985). JANA: A new iterative polyexponential curve stripping program. Comput Methods Programs Biomed 20: 269–275.
Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL (2003). Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci 17: 2212–2218.
Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M et al (2005). Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry 57: 573–582.
Hitzemann R, Curell J, Hom D (1982). Effects of naloxone on d-amphetamine- and apomorphine-induced behavior. Neuropharmacology 21: 1005–1011.
Hurd YL, Ungerstedt U (1989). In vivo neurochemical profile of dopamine uptake inhibitors and releasers in rat caudate-putamen. Eur J Pharmacol 166: 251–260.
Jaffe C, Bush KR, Straits-Troster K, Meredith C, Ronwall L, Rosenbaum G et al (2005). A comparison of methamphetamine-dependent inpatients childhood attention deficit hyperactivity disorder symptomatology. J Addict Dis 24: 133–152.
Jayaram-Lindstrom N, Wennberg P, Hurd YL, Franck J (2004). Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol 24: 665–669.
Jones DN, Holtzman SG (1992). Interaction between opioid antagonists and amphetamine: evidence for mediation by central delta opioid receptors. J Pharmacol Exp Ther 262: 638–645.
Joyce PR, Donald RA (1987). Naloxone augments the hypothalamic–pituitary–adrenal axis response to methylphenidate in normal subjects. J Psychiatr Res 21: 297–300.
Kiyatkin EA, Brown PL (2003). Naloxone depresses cocaine self-administration and delays its initiation on the following day. Neuroreport 14: 251–255.
Kosten T, Silverman DG, Fleming J, Kosten TA, Gawin FH, Compton M et al (1992). Intravenous cocaine challenges during naltrexone maintenance: a preliminary study. Biol Psychiatry 32: 543–548.
Kuzmin AV, Gerrits MA, van Ree JM (1997). Naloxone inhibits the reinforcing and motivational aspects of cocaine addiction in mice. Life Sci 60: 257–264.
Lee MC, Wagner HN, Tanada S, Frost JJ, Bice AN, Dannals RF (1988). Duration of occupancy of opiate receptors. J Nucl Med 29: 1207–1211.
Martin WR, Sloan JW, Sapira JD, Jasinski DR (1971). Physiologic, subjective, and behavioural effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12: 245–258.
Mayfield DG (1973). The effect of intravenous methamphetamine on mood. Int J Addict 8: 565–568.
McNair DM, Lorr M, Droppleman LF (1971). Manual for the Profile of Mood States. Educational and Industrial Testing Service: San Diego, CA.
Meyer MC, Straughn AB, Lo MW (1984). Bioequivalence, dose-proportionality and pharmacokinetics of naltrexone after oral administration. Clin Psychiatry 45: 15–19.
Naber D, Pickar D, Davis GC, Cohen RM, Jimerson DC, Elchisak MA et al (1981). Naloxone effects on beta-endorphin, cortisol, prolactin, growth hormone, HVA and MHPG in plasma of normal volunteers. Psychopharmacology (Berl) 74: 125–128.
O'Brien CP (2005). Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry 162: 1423–1431.
O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B (1992). Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry 49: 881–887.
Oslin DW, Pettinati HM, Volpicelli JR, Wolf AL, Kampman KM, O'Brien CP (1999). The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat 16: 163–167.
Schmitz JM, Stotts AL, Rhoades HM, Grabowski J (2001). Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav 26: 167–180.
Schmitz JM, Stotts AL, Sayre SL, DeLaune KA, Grabowski J (2004). Treatment of cocaine-alcohol dependence with naltrexone and relapse prevention therapy. Am J Addic 13: 333–341.
Sofuoglu M, Singha A, Kosten TR, McCance-Katz FE, Petrakis I, Oliveto A (2003). Effects of naltrexone and isradipine, alone or in combination, on cocaine responses in humans. Pharmacol Biochem Behav 75: 801–808.
Srisurapanont M, Jarusuraisin N, Kittirattanapaiboon P (2001). Treatment for amphetamine dependence and abuse. Cochrane Database Syst Rev CD003022.
Statistical Consultants Inc. (1986). PCNONLIN and NONLIN 84. Software for the statistical analysis of nonlinear models. Am Stat 40: 52.
Tiffany ST, Singleton E, Haertzen CA, Henningfield JE (1993). The development of a cocaine craving questionnaire. Drug Alcohol Depend 34: 19–28.
Trujillo KA, Belluzzi JD, Stein L (1991). Naloxone blockade of amphetamine place preference conditioning. Psychopharmacology (Berl) 104: 265–274.
Trujillo KA, Belluzzi JD, Stein L (1989). Naloxone suppression of self-stimulation is independent of response difficulty. Pharmacol Biochem Behav 33: 147–155.
Vocci F, Ling W (2005). Medications development: successes and challenges. Pharmacol Ther 108: 94–108.
Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP (1992). Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49: 876–880.
Wang JQ, McGinty JF (1995). Alterations in striatal zif/268, preprodynorphin and preproenkephalin mRNA expression induced by repeated amphetamine administration in rats. Brain Res 673: 262–274.
Acknowledgements
We are grateful to Margareta Gard-Hedander and Else-Britt Hillner for handling the study medication and Ms Ingrid Dahlin for handling and management of blood samples. This study was supported by the Swedish Science Council (grant 14645-01A), the Swedish National Drug Policy Coordinator, and the Stockholm County Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
All authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jayaram-Lindström, N., Konstenius, M., Eksborg, S. et al. Naltrexone Attenuates the Subjective Effects of Amphetamine in Patients with Amphetamine Dependence. Neuropsychopharmacol 33, 1856–1863 (2008). https://doi.org/10.1038/sj.npp.1301572
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301572
Keywords
This article is cited by
-
Verification of a genetic locus for methamphetamine intake and the impact of morphine
Mammalian Genome (2018)
-
Naltrexone modulates dopamine release following chronic, but not acute amphetamine administration: a translational study
Translational Psychiatry (2017)
-
Naltrexone moderates the relationship between cue-induced craving and subjective response to methamphetamine in individuals with methamphetamine use disorder
Psychopharmacology (2017)
-
The Effects of Naltrexone on Subjective Response to Methamphetamine in a Clinical Sample: a Double-Blind, Placebo-Controlled Laboratory Study
Neuropsychopharmacology (2015)
-
Agonist Medications for the Treatment of Cocaine Use Disorder
Neuropsychopharmacology (2015)