Abstract
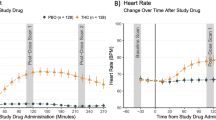
Cannabis is one of the most widely used illicit substances and there is growing interest in the association between cannabis use and psychosis. Delta-9-Tetrahydrocannabinol (Δ-9-THC) the principal active ingredient of cannabis has been shown to induce psychotomimetic and amnestic effects in healthy individuals. Whether people who frequently use cannabis are either protected from or are tolerant to these effects of Δ-9-THC has not been established. In a 3-day, double-blind, randomized, placebo-controlled study, the dose-related effects of 0, 2.5, and 5 mg intravenous Δ-9-THC were studied in 30 frequent users of cannabis and compared to 22 healthy controls. Δ-9-THC (1) produced transient psychotomimetic effects and perceptual alterations; (2) impaired memory and attention; (3) increased subjective effects of ‘high’; (4) produced tachycardia; and (5) increased serum cortisol in both groups. However, relative to controls, frequent users showed blunted responses to the psychotomimetic, perceptual altering, cognitive impairing, anxiogenic, and cortisol increasing effects of Δ-9-THC but not to its euphoric effects. Frequent users also had lower prolactin levels. These data suggest that frequent users of cannabis are either inherently blunted in their response to, and/or develop tolerance to the psychotomimetic, perceptual altering, amnestic, endocrine, and other effects of cannabinoids.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abood ME, Sauss C, Fan F, Tilton CL, Martin BR (1993). Development of behavioral tolerance to delta 9-THC without alteration of cannabinoid receptor binding or mRNA levels in whole brain. Pharmacol Biochem Behav 46: 575–579.
Anthony JC, Trinkoff AM (1989). United States epidemiologic data on drug use and abuse: how are they relevant to testing abuse liability of drugs? NIDA Res Monogr 92: 241–266.
Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (1995). Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab Dispos 23: 825–831.
Brandt J (1991). ‘The hopkins verbal learning test. Development of a new memory test with 6 equivalent forms’. Clin Neuropsychol 5: 125–142.
Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ (1999). Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem 73: 2447–2459.
Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS et al (1998). Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11: 125–136.
Brunner E, Domhof S, Langer F (2002). Nonparametric Analysis of Longitudinal Data in Factorial Experiments. John Wiley and Sons: New York, NY.
Budney AJ, Hughes JR, Moore BA, Vandrey R (2004). Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 161: 1967–1977.
Bylsma FW, Rebok GW, Brandt J (1991). Long-term retention of implicit learning in Huntington's disease. Neuropsychologia 29: 1213–1221.
Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H et al (2005). Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 57: 1117–1127.
Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS (2004). Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA 291: 2114–2121.
Conrod PJ, Peterson JB, Pihl RO (2001). Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology (Berl) 157: 20–30.
de Miguel R, Romero J, Munoz RM, Garcia-Gil L, Gonzalez S, Villanua MA et al (1998a). Effects of cannabinoids on prolactin and gonadotrophin secretion: involvement of changes in hypothalamic gamma-aminobutyric acid (GABA) inputs. Biochem Pharmacol 56: 1331–1338.
de Miguel R, Romero J, Munoz RM, Garcia-Gil L, Gonzalez S, Villanua MA et al (1998b). Effects of cannabinoids on prolactin and gonadotrophin secretion: involvement of changes in hypothalamic gamma-aminobutyric acid (GABA) inputs. Biochem Pharmacol 56: 1331–1338.
Di Marzo V, Berrendero F, Bisogno T, Gonzalez S, Cavaliere P, Romero J et al (2000). Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of delta9-tetrahydrocannabinol-tolerant rats. J Neurochem 74: 1627–1635.
D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT et al (2004). The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29: 1558–1572.
D'Souza DC (2007). Cannabinoids and psychosis. Int Rev Neurobiol 78: 289–326.
Eissenberg T, Balster RL (2000). Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend 59 (Suppl 1): S41–S60.
Fergusson DM, Horwood LJ, Swain-Campbell NR (2003b). Cannabis dependence and psychotic symptoms in young people. Psychol Med 33: 15–21.
Fergusson DM, Horwood LJ, Lynskey MT, Madden PA (2003a). Early reactions to cannabis predict later dependence. Arch Gen Psychiatry 60: 1033–1039.
First MB, Spitzer RL, Gibbon M, Williams JBW (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders Non-patient edition. In: American Psychiatric Association, Washington, DC.
Gonzalez S, Manzanares J, Berrendero F, Wenger T, Corchero J, Bisogno T et al (1999). Identification of endocannabinoids and cannabinoid CB(1) receptor mRNA in the pituitary gland. Neuroendocrinology 70: 137–145.
Gonzalez S, Cebeira M, Fernandez-Ruiz J (2005). Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 81: 300–318.
Gordon M (1986). Microprocessor-based assessment of attention deficit disorders (ADD). Psychopharmacol Bull 22: 288–290.
Green B, Kavanagh D, Young R (2003). Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev 22: 453–460.
Guimaraes FS, de Aguiar JC, Mechoulam R, Breuer A (1994). Anxiolytic effect of cannabidiol derivatives in the elevated plus-maze. Gen Pharmacol 25: 161–164.
Haertzen CA (1965). Addiction Research Center Inventory (ARCI): development of a general drug estimation scale. J Nerv Ment Dis 141: 300–307.
Haertzen CA (1966). Development of scales based on patterns of drug effects, using the addiction Research Center Inventory (ARCI). Psychol Rep 18: 163–194.
Hall W, Degenhardt L, Teesson M (2004). Cannabis use and psychotic disorders: an update. Drug Alcohol Rev 23: 433–443.
Hampson RE, Simeral JD, Kelly EJ, Deadwyler SA (2003). Tolerance to the memory disruptive effects of cannabinoids involves adaptation by hippocampal neurons. Hippocampus 13: 543–556.
Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW (1999a). Abstinence symptoms following oral THC administration to humans. Psychopharmacology 141: 385–394.
Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW (1999b). Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 141: 395–404.
Harclerode J (1984). Endocrine effects of marijuana in the male: preclinical studies. In: Braude MC and Ludford JP (eds). NIDA Research Monograph Series. National Institute on Drug Abuse: Rockville, MD, pp 45–114.
Henquet C, Rosa A, Krabbendam L, Papiol S, Fananas L, Drukker M et al (2006a). An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 31: 2748–2757.
Henquet C, Rosa A, Krabbendam L, Papiol S, Fananás L, Drukker M et al (2006b). An experimental study of catechol-O-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 31: 2748–2757.
Henquet C, Murray R, Linszen D, van Os J (2005). The environment and schizophrenia: the role of cannabis use. Schizophr Bull 31: 608–612.
Hollister LE (1988). Cannabis--1988. Acta Psychiatr Scand Suppl 345: 108–118.
Hopfer CJ, Lessem JM, Hartman CA, Stallings MC, Cherny SS, Corley RP et al (2007). A genome-wide scan for loci influencing adolescent cannabis dependence symptoms: evidence for linkage on chromosomes 3 and 9. Drug Alcohol Depend 89: 34–41.
Hopfer CJ, Young SE, Purcell S, Crowley TJ, Stallings MC, Corley RP et al (2006). Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. Am J Med Genet B Neuropsychiatr Genet 141: 895–901.
Jones RT, Benowitz N, Bachman J (1976). Clinical studies of cannabis tolerance and dependence. Ann N Y Acad Sci 282: 221–239.
Jones RT, Benowitz NL, Herning RI (1981). Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol 21: 143S–152S.
Kandel DB, Chen K (2000). Types of marijuana users by longitudinal course. J Stud Alcohol 61: 367–378.
Kay SR, Opler LA, Lindenmayer JP (1989). The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry-Suppl 59–67.
Kelleher LM, Stough C, Sergejew AA, Rolfe T (2004). The effects of cannabis on information-processing speed. Addict Behav 29: 1213–1219.
Leweke FM, Giuffrida A, Koethe D, Schreiber D, Nolden BM, Kranaster L et al (2007). Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res 94: 29–36.
Leweke FM, Gerth CW, Klosterkotter J (2004). Cannabis-associated psychosis: current status of research. CNS Drugs 18: 895–910.
Leweke FM, Schneider U, Radwan M, Schmidt E, Emrich HM (2000). Different effects of nabilone and cannabidiol on binocular depth inversion in Man. Pharmacol Biochem Behav 66: 175–181.
Leweke FM, Schneider U, Thies M, Munte TF, Emrich HM (1999). Effects of synthetic delta9-tetrahydrocannabinol on binocular depth inversion of natural and artificial objects in man. Psychopharmacology (Berl) 142: 230–235.
Leweke FM (2007). Cannabidiol as an antipsychotic—new perspectives. In: 2nd International Cannabis and Mental Health Conference. Institute of Psychiatry: London, UK.
Lichtman AH, Martin BR (2005). Cannabinoid tolerance and dependence. Handb Exp Pharmacol 168: 691–717. Review.
Lichtman AH, Varvel SA, Martin BR (2002). Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fatty Acids 66: 269–285.
Lyons MJ, Toomey R, Meyer JM, Green AI, Eisen SA, Goldberg J et al (1997). How do genes influence marijuana use? The role of subjective effects. Addiction 92: 409–417.
Martin BR, Sim-Selley LJ, Selley DE (2004). Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci 25: 325–330.
Mechoulam R, Ben-Shabat S (1999). From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: the ongoing story of cannabis. Nat Prod Rep 16: 131–143.
Murphy LL, Munoz RM, Adrian BA, Villanua MA (1998a). Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis 5: 432–446.
Murphy LL, Munoz RM, Adrian BA, Villanua MA (1998b). Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis 5: 432–446.
Newlin DB, Thomson JB (1990). Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull 108: 383–402.
Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R (2006). The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27: 73–100.
Pertwee RG, Stevenson LA, Griffin G (1993). Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. [erratum appears in Br J Pharmacol 1994 Mar; 111(3):968]. Br J Pharmacol 110: 1483–1490.
Pollock VE (1992). Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Am J Psychiatry 149: 1534–1538.
Rodriguez De Fonseca F, Fernandez-Ruiz JJ, Murphy LL, Cebeira M, Steger RW, Bartke A et al (1992). Acute effects of delta-9-tetrahydrocannabinol on dopaminergic activity in several rat brain areas. Pharmacol, Biochem Behav 42: 269–275.
Rodriguez de Fonseca F, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA (1994). Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav 47: 33–40.
Romero J, Berrendero F, Manzanares J, Perez A, Corchero J, Fuentes JA et al (1998). Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse 30: 298–308.
Romero J, Garcia L, Fernandez-Ruiz JJ, Cebeira M, Ramos JA (1995). Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-tetrahydrocannabinol. Pharmacol Biochem Behav 51: 731–737.
Rubino T, Vigano D, Massi P, Parolaro D (2000a). Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55,940. J Neurochem 75: 2080–2086.
Rubino T, Vigano D, Massi P, Spinello M, Zagato E, Giagnoni G et al (2000b). Chronic delta-9-tetrahydrocannabinol treatment increases cAMP levels and cAMP-dependent protein kinase activity in some rat brain regions. Neuropharmacology 39: 1331–1336.
Rubino T, Patrini G, Parenti M, Massi P, Parolaro D (1997). Chronic treatment with a synthetic cannabinoid CP-55,940 alters G-protein expression in the rat central nervous system. Brain Res Mol Brain Res 44: 191–197.
Russo EB, McPartland JM (2003). Cannabis is more than simply delta(9)-tetrahydrocannabinol. [comment]. Psychopharmacology 165: 431–432; author reply 433–434.
SAMHSA (2004). Results from the 2003 national survey on drug use and health: national findings. Substance Abuse and Mental Health Services Administration: Rockville, MD.
Schuckit MA, Greenblatt D, Gold E, Irwin M (1991b). Reactions to ethanol and diazepam in healthy young men. J Stud Alcohol 52: 180–187.
Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger Jr JI (1996). Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol 57: 368–377.
Schuckit MA, Duthie LA, Mahler HI, Irwin M, Monteiro MG (1991a). Subjective feelings and changes in body sway following diazepam in sons of alcoholics and control subjects. J Stud Alcohol 52: 601–608.
Schuckit MA, Smith TL, Anderson KG, Brown SA (2004). Testing the level of response to alcohol: social information processing model of alcoholism risk--a 20-year prospective study. Alcohol Clin Exp Res 28: 1881–1889.
Schuckit MA (1985a). Behavioral effects of alcohol in sons of alcoholics. Recent Dev Alcohol 3: 11–19.
Schuckit MA (1985c). Ethanol-induced changes in body sway in men at high alcoholism risk. Arch Gen Psychiatry 42: 375–379.
Schuckit MA (2000). Genetics of the risk for alcoholism. Am J Addict 9: 103–112.
Schuckit MA (1994). Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151: 184–189.
Schuckit MA (1985b). The clinical implications of primary diagnostic groups among alcoholics. Arch Gen Psychiatry 42: 1043–1049.
Sim-Selley LJ, Martin BR (2002). Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther 303: 36–44.
Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR . et al (2006). Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol 70: 986–996.
Slosson RL (1963). Slosson Intelligence Test (SIT) for Children and Adults. In: Slosson Educational Publications: East Aurora, NY.
Sobell LC, Sobell MB (1992). Timeline follow-back: a technique for assessing self-reported alcohol consumption. Litten AR, Totowa J (eds). Measuring Alcohol Consumption. The Humana Press: NJ, USA.
Solowij N (1998). Cannabis and Cognitive Functioning. Cambridge University Press: Cambridge.
Solowij N (1995). Do cognitive impairments recover following cessation of cannabis use? Life Sci 56: 2119–2126.
Stinson FS, Ruan WJ, Pickering R, Grant BF (2006). Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med 36: 1447–1460.
Verdoux H, Tournier M (2004). Cannabis use and risk of psychosis: an etiological link? Epidemiologia e Psichiatria Sociale 13: 113–119.
Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H (2002). Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 161: 331–339.
Weiser M, Noy S (2005). Interpreting the association between cannabis use and increased risk for schizophrenia. Dialogues Clin Neurosci 7: 81–85.
Whitlow CT, Freedland CS, Porrino LJ (2003). Functional consequences of the repeated administration of Delta9-tetrahydrocannabinol in the rat. Drug Alcohol Depend 71: 169–177.
Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG (1982). Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology 76: 245–250.
Zuardi AW, Rodrigues JA, Cunha JM (1991). Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology 104: 260–264.
Zuardi AW, Morais SL, Guimaraes FS, Mechoulam R (1995). Antipsychotic effect of cannabidiol [letter]. J Clin Psychiatry 56: 485–486.
Acknowledgements
We thank Dr George Kottayil (Unimed Pharmaceuticals Inc.) for providing Δ-9-THC. We acknowledge the critical clinical research contributions of the Biological Studies Unit, VA Connecticut Healthcare System including Elizabeth O'Donell RN; Angelina Genovese RN; Sonah Yoo RPh; Robert Sturwold RPh, and Mr Willie Ford. This study was supported by National Institute of Drug Abuse (DA12382-01) to DCD in addition to indirect support from the 1) Department of Veterans Affairs (Schizophrenia Biological Research Center, Alcohol Research Center, National Center for PTSD (John Krystal)), 2) National Institute of Mental Health (MH61019-02 to DCD).
Author information
Authors and Affiliations
Corresponding author
Additional information
CONFLICT OF INTEREST
There are no direct or indirect conflicts of interest for any of the authors relevant to the subject of this article.
Rights and permissions
About this article
Cite this article
D'Souza, D., Ranganathan, M., Braley, G. et al. Blunted Psychotomimetic and Amnestic Effects of Δ-9-Tetrahydrocannabinol in Frequent Users of Cannabis. Neuropsychopharmacol 33, 2505–2516 (2008). https://doi.org/10.1038/sj.npp.1301643
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301643
Keywords
This article is cited by
-
Effects of acute cannabis inhalation on reaction time, decision-making, and memory using a tablet-based application
Journal of Cannabis Research (2024)
-
The Effects of Chronic Marijuana Administration on 6-OHDA-Induced Learning & Memory Impairment and Hippocampal Dopamine and Cannabinoid Receptors Interaction in Male Rats
Neurochemical Research (2023)
-
Rates and correlates of cannabis-associated psychotic symptoms in over 230,000 people who use cannabis
Translational Psychiatry (2022)
-
Sex-specific mechanisms of tolerance for the cannabinoid agonists CP55,940 and delta-9-tetrahydrocannabinol (Δ9-THC)
Psychopharmacology (2022)
-
Factors that Impact the Pharmacokinetic and Pharmacodynamic Effects of Cannabis: a Review of Human Laboratory Studies
Current Addiction Reports (2022)