Abstract
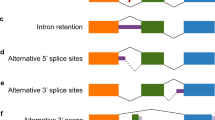
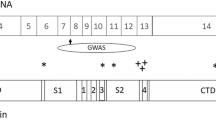
Genetic variation in the metabotropic glutamate receptor 3 (GRM3, mGluR3) has been associated with schizophrenia, but the mechanism by which it confers risk is unknown. Previously, we reported the existence of a splice variant, GRM3Δ4, which has an exon 4 deletion and encodes a truncated form of the receptor that is expressed in brain. The aim of the present study was to determine whether expression of this splice variant is altered in individuals with schizophrenia and is affected by a risk genotype. We measured GRM3 and GRM3Δ4 transcripts in human dorsolateral prefrontal cortex (DLPFC) and hippocampus of the CBDB/NIMH collection (∼70 controls, ∼30 schizophrenia patients) and in the DLPFC of the Stanley Array Collection. Expression data of GRM3 mRNA in the DLPFC were inconsistent: GRM3 was increased in schizophrenia patients in the CBDB/NIMH collection, but not in the Stanley Array Collection. GRM3 expression did not change in the frontal cortex of rats treated chronically with haloperidol or clozapine. An exon 3 SNP previously associated with schizophrenia (rs2228595) predicted increased expression of the GRM3Δ4 splice variant. Our results suggest that rs2228595, or a neighboring SNP in linkage disequilibrium with it, may contribute to risk for schizophrenia by modulating GRM3 splicing.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Battaglia G, Bruno V, Ngomba RT, Di Grezia R, Copani A, Nicoletti F (1998). Selective activation of group-II metabotropic glutamate receptors is protective against excitotoxic neuronal death. Eur J Pharmacol 356: 271–274.
Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR (2003). ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res 31: 3568–3571.
Chen Q, He G, Chen Q, Wu S, Xu Y, Feng G et al (2005). A case–control study of the relationship between the metabotropic glutamate receptor 3 gene and schizophrenia in the Chinese population. Schizophr Res 73: 21–26.
Comings DE, MacMurray JP (2000). Molecular heterosis: a review. Mol Genet Metab 71: 19–31.
Conn PJ, Pin JP (1997). Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–237.
Corti C, Crepaldi L, Mion S, Roth AL, Xuereb JH, Ferraguti F et al (2007). Altered dimerization of metabotropic glutamate receptor 3 in schizophrenia. Biol Psychiatry 62: 747–755.
Crook JM, Akil M, Law BC, Hyde TM, Kleinman JE (2002). Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann's area 46 of the dorsolateral prefrontal cortex from patients with schizophrenia and normal subjects. Mol Psychiatry 7: 157–164.
de Quervain DJ, Papassotiropoulos A (2006). Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc Natl Acad Sci USA 103: 4270–4274.
East SJ, Hill MP, Brotchie JM (1995). Metabotropic glutamate receptor agonists inhibit endogenous glutamate release from rat striatal synaptosomes. Eur J Pharmacol 277: 117–121.
Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR (2004). Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci USA 101: 12604–12609.
Fairbrother WG, Yeh RF, Sharp PA, Burge CB (2002). Predictive identification of exonic splicing enhancers in human genes. Science 297: 1007–1013.
Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA et al (2005). Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case–parent trios. Am J Hum Genet 77: 918–936.
Fujii Y, Shibata H, Kikuta R, Makino C, Tani A, Hirata N et al (2003). Positive associations of polymorphisms in the metabotropic glutamate receptor type 3 gene (GRM3) with schizophrenia. Psychiatr Genet 13: 71–76.
Green E, Grozeva D, Norton N, Jones I, Jones L, O'Donovan MC et al (2006). Variation at GRM3 influences susceptibility to psychotic bipolar disorder [Abstract]. Am J Med Genet B Neuropsychiatr Genet 141B: 683–824.
Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH (2005). Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 57: 123–131.
Harrison PJ, Weinberger DR (2005). Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10: 40–68; image 5.
Javitt DC (2004). Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry 9: 984–997, 979.
Krystal JH, Abi-Saab W, Perry E, D'Souza DC, Liu N, Gueorguieva R et al (2005). Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 179: 303–309.
Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R et al (2006). Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA 103: 6747–6752.
Lipska BK, Deep-Soboslay A, Shannon Weickert C, Hyde TM, Martin CE, Herman MM et al (2006a). Critical factors in gene expression in postmortem human brain: focus on studies in schizophrenia. Biol Psychiatry 60: 650–658.
Lipska BK, Peters T, Hyde TM, Halim N, Horowitz C, Mitkus S et al (2006b). Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Hum Mol Genet 15: 1245–1258.
Lovinger DM, McCool BA (1995). Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol 73: 1076–1083.
Marti SB, Cichon S, Propping P, Nothen M (2002). Metabotropic glutamate receptor 3 (GRM3) gene variation is not associated with schizophrenia or bipolar affective disorder in the German population. Am J Med Genet 114: 46–50.
Moghaddam B, Adams BW (1998). Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats.[comment]. Science 281: 1349–1352.
Norton N, Williams HJ, Dwyer S, Ivanov D, Preece AC, Gerrish A et al (2005). No evidence for association between polymorphisms in GRM3 and schizophrenia. BMC Psychiatry 5: 23.
Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC (1998). Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Brain Res Mol Brain Res 56: 207–217.
Olszewski RT, Bukhari N, Zhou J, Kozikowski AP, Wroblewski JT, Shamimi-Noori S et al (2004). NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem 89: 876–885.
Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV et al (2007). Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13: 1102–1107.
Richardson-Burns SM, Haroutunian V, Davis KL, Watson SJ, Meador-Woodruff JH (2000). Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol Psychiatry 47: 22–28.
Sartorius LJ, Nagappan G, Lipska BK, Lu B, Sei Y, Ren-Patterson R et al (2006). Alternative splicing of human metabotropic glutamate receptor 3. J Neurochem 96: 1139–1148.
Schwab SG, Plummer C, Doyle A, Albus M, Borrman-Hassenbach M, Maier W et al (2006). Association with schizophrenia in three candidate genes: GRM-3, AKT-1 and NRG-1 [Abstract]. Am J Med Genet B Neuropsychiatr Genet 141B: 683–824.
Straub RE, Weinberger DR (2006). Schizophrenia genes—famine to feast. Biol Psychiatry 60: 81–83.
Tascedda F, Blom JM, Brunello N, Zolin K, Gennarelli M, Colzi A et al (2001). Modulation of glutamate receptors in response to the novel antipsychotic olanzapine in rats. Biol Psychiatry 50: 117–122.
Tochigi M, Suga M, Ohashi J, Otowa T, Yamasue H, Kasai K et al (2006). No association between the metabotropic glutamate receptor type 3 gene (GRM3) and schizophrenia in a Japanese population [Abstract]. Am J Med GenetB Neuropsychiatr Genet 141B: 683–824.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A et al (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034.
Yoshino M, Sawada S, Yamamoto C, Kamiya H (1996). A metabotropic glutamate receptor agonist DCG-IV suppresses synaptic transmission at mossy fiber pathway of the guinea pig hippocampus. Neurosci Lett 207: 70–72.
Acknowledgements
LJS is a student in the NIH/Oxford Graduate Partnership Program. This research was supported by the Intramural Research Program of the NIH, NIMH. Post-mortem brain tissue was donated by the Stanley Medical Research Institute, courtesy of Drs Michael B Knable, E Fuller Torrey, Maree J Webster, Serge Weis, and Robert H Yolken. We thank Ms Cara Horowitz, Ms Tricia Peters, and Mr Krishna Vakkalanka for their excellent technical assistance, Dr Mary M Herman for her neuropathological assessments, and Amy Deep-Soboslay for her work on the demographic and clinical data. We thank the staff of the Offices of the Chief Medical Examiner of District of Columbia and of Northern Virginia for their assistance. We also thank the families of the deceased, who generously donated brain tissue as well as their time and effort to make this study possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
Dr Harrison's research is supported by the United Kingdom Medical Research Council, Stanley Medical Research Institute, National Alliance for Research on Schizophrenia and Depression, Wellcome Trust, and an unrestricted grant from GlaxoSmithKline. In the past 3 years, Dr Harrison has received honoraria for giving educational lectures or chairing scientific meetings from Bristol-Meyers Squibb, GlaxoSmithKline, Janssen, Lilly, Merck, Sanofi, and Servier pharmaceutical companies, and has been an adviser to Curidium, Janssen, and Wyeth. The other authors declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Sartorius, L., Weinberger, D., Hyde, T. et al. Expression of a GRM3 Splice Variant is Increased in the Dorsolateral Prefrontal Cortex of Individuals Carrying a Schizophrenia Risk SNP. Neuropsychopharmacol 33, 2626–2634 (2008). https://doi.org/10.1038/sj.npp.1301669
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301669
Keywords
This article is cited by
-
Variation Screening of Zygote Arrest 1(ZAR1) in Women with Recurrent Zygote Arrest During IVF/ICSI Programs
Reproductive Sciences (2020)
-
A Novel Ultrasensitive In Situ Hybridization Approach to Detect Short Sequences and Splice Variants with Cellular Resolution
Molecular Neurobiology (2018)
-
mGluR2/3 mechanisms in primate dorsolateral prefrontal cortex: evidence for both presynaptic and postsynaptic actions
Molecular Psychiatry (2017)
-
Meta-analysis supports GWAS-implicated link between GRM3 and schizophrenia risk
Translational Psychiatry (2017)
-
Contrasting changes in DRD1 and DRD2 splice variant expression in schizophrenia and affective disorders, and associations with SNPs in postmortem brain
Molecular Psychiatry (2014)