Abstract
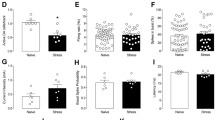
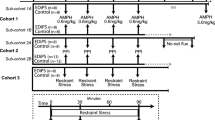
Previously we have shown that twelve weeks of repeated low-dose d-amphetamine (AMPH) exposure in rhesus monkeys induces a long-lasting enhancement of behavioral responses to acute low-dose challenge. The present study was designed to investigate the behavioral and neurochemical consequences of a six-week regimen of low-dose AMPH exposure (0.1–1.0 mg/kg, i.m., b.i.d.) in rhesus monkeys. SPECT imaging of AMPH's (0.4 mg/kg) ability to displace [123I]IBZM bound to D2 dopamine receptors in the striatum of saline control and AMPH-treated animals prior to and following chronic treatment was accomplished using a bolus/constant infusion paradigm. Following chronic AMPH treatment, all monkeys showed an enhanced behavioral response to acute AMPH challenge and a significant decrease in the percent of AMPH-induced displacement of [123I]IBZM in striatum compared to their pretreatment scans. These findings suggest that relatively small changes in presynaptic dopamine function may be reflected in significant alterations in the behavioral response to acute AMPH challenge.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M . (1998): Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am J Psychiatry 155 (6): 761–767
Baldwin RM, Zea-Ponce Y, Zoghbi SS, Al-Tikriti MS, Seibyl JP, Sybirska EH, Malison RT, Laruelle M, Charney DS, Hoffer PB, Innis RB . (1994): [123I]IBF, [123I]epidepride, and [123I]2′-ISP in nonhuman primates. Nucl Med Biol 21: 969–976
Bickerdike MJ, Abercrombie ED . (1997): Striatal acetylcholine release correlates with behavioral sensitization in rats withdrawn from chronic amphetamine. J Pharmacol Exp Ther 282 (2): 818–826
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D . (1997): Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 94 (6): 2569–2574
Castner SA, Goldman-Rakic PS . (1999): Long-lasting psychotomimetic consequences of repeated low dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology 20 (1): 10–28
Duman RS, Tallman JF, Nestler EJ . (1988): Acute and chronic opiate-regulation of adenylate cyclase in brain: Specific effects in locus coeruleus. J Pharmacol Exper Ther 246 (3): 1033–1039
Ellison G, Eison MS, Huberman HS, Daniel F . (1978): Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science 201 (4352): 276–278
Ellison G, Ratan R . (1982): The late stage following continuous amphetamine administration to rats is correlated with altered dopamine but not serotonin metabolism. Life Sci 31 (8): 771–777
Holman BL, Carvalho PA, Zimmerman RE, Johnson KA, Tumeh SS, Smith AP, Genna S . (1990): Brain perfusion SPECT using an annular single crystal camera: Initial clinical experience. J Nucl Med 31 (9): 1456–1461
Kalivas PW, Stewart J . (1991): Dopamine transmission in the initiation and expression of drug and stress induced sensitization of motor activity. Brain Res Rev 16: 223–244
Kuczenski R, Segal D . (1989): Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci 9 (6): 2051–2065
Kuczenski R, Segal DS . (1990): In vivo measures of monoamines during amphetamine-induced behaviors in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 14(suppl):S37–S50
Kuczenski R, Segal DS, Aizenstein ML . (1991): Amphetamine, cocaine, and fencamfamine: Relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci 11 (9): 2703–2712
Kung MP, Kung HF . (1989): Peracetic acid as a superior oxidant for preparation of [123I]IBZM: A potential dopamine D-2 receptor imaging agent. J Label Compound Radiopharmacol 27: 691–700
Laruelle M, Abi-Dargham A, Al-Tikriti MS, Baldwin RM, Zea Ponce Y,, Zoghbi SS, Charney DS, Hoffer PB, Innis RB . (1994a): SPECT quantification of 123I-Iomazenil binding to bezodiazepine receptors in nonhuman primates. II. Equilibrium analysis of constant infusion experiments and correlation with in vitro parameters. J Cerb Blood Flow Metab 14: 453–465
Laruelle M, Al-Tikriti MS, Zea Ponce Y, Zoghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung HF, Innis RB . (1994b): In vivo quantification of dopamine D2 receptors parameters in nonhuman primates with 123I-iodobenzofuran and single photon emission computarized tomography. Eur J Pharmacol 263: 39–51
Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB . (1996): Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 93 (17): 9235–9240
Laruelle M, Iyer RN, Al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, Baldwin RM, Kung HF, Charney DS, Hoffer PB, Innis RB, Bradberry CW . (1997): Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 25 (1): 1–14
Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME . (1997): Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res 766(1–2):113–120
Miserendino MJ, Nestler EJ . (1995): Behavioral sensitization to cocaine: Modulation by the cyclic AMP system in the nucleus accumbens. Brain Res 674 (2): 299–306
Nestler EJ . (1993): Cellular responses to chronic treatment with drugs of abuse. Crit Rev Neurobiol 7 (1): 23–39
Nestler EJ, Aghajanian GK . (1997): Molecular and cellular basis of addiction. Science 278 (5335): 58–63
Paulson PE, Camp DM, Robinson TE . (1991): Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology 103 (4): 480–492
Proakis AG, Harris GB . (1978): Comparative penetration of glycopyrrolate and atropine across the blood-brain and placental barriers in anesthetized dogs. Anesthesiology 48 (5): 339–344
Ridley RM, Baker HF, Owen F, Cross AJ, Crow TJ . (1983): Behavioural and biochemical effects of chronic treatment with amphetamine in the vervet monkey. Neuropharmacology 22 (4): 551–554
Robinson TE, Becker JB . (1986): Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res 396 (2): 157–198
Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ . (1991): A general role for adaptations in G-proteins and the cyclic AMP system in mediating the actions of morphine and cocaine on neuronal function. Brain Res 548(1–2):100–110
Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA . (1998): Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: Evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci 18 (1): 419–427
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N . (1997): Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386 (6627): 830–833
Williams SM, Goldman-Rakic PS . (1998): Widespread origin of the primate mesofrontal dopamine system. Cerebr Cortex 8: 321–345
Zubal IG, Harrell C, Woods SW, Innis RB, Hoffer PB, Caride VJ, Zimmermann RE . (1990): Comparison of quantitation linearity of three brain SPECT imaging instruments using Tc-99m and 123I. J Nucl Med 31: 769–770
Acknowledgements
The authors would like to thank Heather Findlay, Terri Beattie, Jill Davenport, Lynn Pantages-Torrok, and Louis Amici for their expert technical assistance with SPECT experiments. We also thank Jonathon Traupman for developing the MonkeyWatcher program. This work was supported by an NIMH grant MH44866 awarded to P.S. Goldman-Rakic and the VA Schizophrenia Research Center.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Castner, S., Al-Tikriti, M., Baldwin, R. et al. Behavioral Changes and [123I]IBZM Equilibrium SPECT Measurement of Amphetamine-Induced Dopamine Release in Rhesus Monkeys Exposed to Subchronic Amphetamine. Neuropsychopharmacol 22, 4–13 (2000). https://doi.org/10.1016/S0893-133X(99)00080-9
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1016/S0893-133X(99)00080-9
Keywords
This article is cited by
-
Dopaminergic System Dysfunction in Recreational Dexamphetamine Users
Neuropsychopharmacology (2015)
-
Long-Term Exposure to Oral Methylphenidate or dl-Amphetamine Mixture in Peri-Adolescent Rhesus Monkeys: Effects on Physiology, Behavior, and Dopamine System Development
Neuropsychopharmacology (2012)
-
Amphetamine Sensitization Alters Dendritic Morphology in Prefrontal Cortical Pyramidal Neurons in the Non-Human Primate
Neuropsychopharmacology (2007)
-
Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction
Psychopharmacology (2007)