Abstract
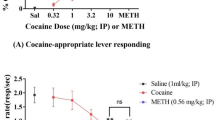
The aim of this study was to examine the effects of perinatal lead exposure on locomotor responding following acute and repeated cocaine challenges (sensitization). Adult female rats were gavaged daily with 0, 8, or 16 mg lead acetate for 30 days prior to breeding. This exposure regimen was maintained throughout gestation and lactation (perinatal exposure). On Day 21, male pups were weaned and lead exposure was discontinued for the remainder of the study. Beginning on postnatal day (PND) 30 or PND 90, and continuing for 14 successive days, separate groups of perinatally-exposed animals were presented with challenges of 10 mg/kg cocaine HCl (i.p.), and tested for locomotor responding. Following this testing period, dose-effect profiles were determined, with animals receiving daily injections of 0, 10, 20, and 40 mg/kg cocaine. The results indicated that both at PND 30 and PND 90 lead-exposed animals were less responsive to the initial administration of cocaine, but exhibited a supersensitivity to the stimulatory effects associated with repeated administration of cocaine, i.e., behavioral sensitization to cocaine was augmented by perinatal lead exposure. Analyses of blood lead levels following the completion of testing revealed that lead levels were below detectable limits for all animals (< 1 μg/dl). Collectively, these findings show that developmental lead contamination produces changes in cocaine sensitivity long after exposure has been discontinued and the toxicant has gained clearance from blood.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH . (1997): Sex differences in dopamine receptor overproduction and elimination. NeuroReport 8: 1495–1498
Banks EC, Ferretti LE, Shucard DW . (1997): Effects of low-level lead exposure on cognitive function in children: A review of behavioral, neuropsychological, and biological evidence. Neurotoxicology 18: 237–282
Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M . (1987): Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med 316: 1037–1043
Brody DJ, Pirkle JL, Kramer RA, Flegal KM, Matte TD, Gunter EW, Paschal DC . (1994): Blood lead levels in the US population: Phase 1 of the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1991). JAMA 272: 277–283
Burkey RT, Nation JR, Grover CA, Bratton GR . (1997): Effects of chronic lead exposure on cocaine-induced disturbance of fixed-interval behavior. Pharmacol Biochem Behav 56: 117–121
Dow-Edwards DL . (1989): Long-term neurochemical and neurobehavioral consequences of cocaine abuse during pregnancy. Ann NY Acad Sci 562: 280–289
Goldman LR, Carra J . (1994): Childhood lead poisoning in 1994. JAMA 272: 315–316
Grover CA, Nation JR, Bratton GR . (1993): Chronic exposure to lead attenuates cocaine-induced behavioral activation. Pharmacol Biochem Behav 44: 221–225
Hemby SE, Co C, Dworkin SI, Smith JE . (1999): Synergistic elevation in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (speedball) in rats. J Pharmacol Exper Therap 288: 274–280
Holson RR, Pearce B . (1992): Principals and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol 14: 221–228
Lasley SM, Lane JD . (1988): Diminished regulation of mesolimbic dopaminergic activity in the rat after chronic lead exposure. Toxicol Appl Pharmacol 95: 474–483
Laviola G, Wood RD, Kuhn C, Francis R, Spear L . (1995): Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exper Therap 275: 345–357
Mello N, Negus S, Lukas SE, Mendelson JH, Sholar JW, Drieze J . (1995): A primate model of polydrug abuse: Cocaine and heroin combinations. J Pharmacol Exper Therap 274: 1325–1337
Mielke HW . (1999): Lead in the inner cities. Am Sci 87: 62–73
Nation JR, Burkey RT . (1994): Attenuation of cocaine-induced elevation of nucleus accumbens dopamine in lead-exposed rats. Brain Res Bull 35: 101–104
Nation JR, Livermore CL, Bratton GR . (1995): Cadmium exposure attenuates the initiation of behavioral sensitization to cocaine. Brain Res 702: 223–232
Nation JR, Livermore CL, Burkey RT . (1996): Chronic lead exposure attenuates sensitization to the locomotor stimulating effects of cocaine. Drug Alcoh Depend 41: 143–149
Nation JR, Wellman PJ, Livermore CL, Miller DK, Bratton GR . (1997): Brain and plasma levels of cocaine and benzoylecgonine in lead-exposed and cadmium-exposed rats following acute or chronic intraperitoneal administration of cocaine. Toxicol Lett 92: 47–57
Needleman HL, Reiss JA, Tobin MJ, Biesecker GE, Greenhouse JD . (1996): Blood lead levels and delinquent behavior. JAMA 275: 363–369
Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD . (1994): The decline in blood lead levels in the United States. JAMA 272: 284–291
Pokora MJ, Richfield EK, Cory-Slechta DA . (1996): Preferential vulnerability of nucleus accumbens dopamine sites to low-level lead exposure: Time course of effects and interactions with chronic dopamine agonist treatment. J Neurochem 67: 1540–1550
Post RM, Lockfield A, Squillace KM, Contel NR . (1981): Drug-environment interaction: Context dependency of cocaine-induced behavioral sensitization. Toxicol Appl Pharmacol 81: 755–760
Post RM, Weiss SRB, Pert A . (1992): Sensitization and kindling effects of chronic cocaine administration. In Lakoski JM, Galloway MP, White FJ (eds), Cocaine: Pharmacology, Physiology, and Clinical Strategies. Boca Raton, CRC Press, pp 115–161
Rice DC . (1996): Behavioral effects of lead: Commonalties between experimental and epidemiological data. Envir Health Perspect 104: 337–351
Robinson TE, Berridge KC . (1993): The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev 18: 247–291
Rosen JB, Young AM, Beuthin FC, Louis-Ferdinand RT . (1986): Discriminative stimulus properties of amphetamine and other stimulants in lead-exposed and normal rats. Pharmacol Biochem Behav 24: 211–215
Scalzo FM, Ali SF, Frambes NA, Spear LP . (1990): Weaning rats exposed prenatally to cocaine exhibit an increase in striatal D2 binding associated with an increase in ligand affinity. Pharmacol Biochem Behav 37: 371–373
Segal DS, Schuckit MA . (1983): Animal models of stimulant-induced psychosis. In Creese I (ed), Stimulants: Neurochemical, Behavioral, and Clinical Perspectives. New York, Raven Press, pp 131–167
Silbergeld EK, Goldberg AM . (1980): Problems in experimental studies of lead poisoning. In Singhal RL, Thomas JA (eds), Lead Toxicity. Baltimore, MD, Urban and Schwartzberg, pp 19–41
Teicher MH, Andersen SL, Hostetter JC . (1995): Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev Brain Res 89: 167–172
Teicher MH, Dumont NL, Andersen SL . (1998): The developing prefrontal cortex: Is there a transient interneuron that stimulates catecholamine terminals? Synapse 29: 89–91
Widzowski DV, Finkelstein JN, Pokara MJ, Cory-Slechta DA . (1994): Time course of postnatal lead-induced changes in dopamine receptors and their relationship to changes in dopamine sensitivity. Neurotoxicology 15: 853–866
Wise RA, Bozarth MA . (1987): A psychomotor stimulant theory of addiction. Psychol Rev 94: 469–492
Wolf ME, Dahlin SL, Hu X, White K . (1995): Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: Comparison with N-methyl-D-aspartate antagonists. Neuroscience 69: 417–439
Zenick H, Goldsmith M . (1981): Drug discrimination learning in lead-exposed rats. Science 212: 569–571
Acknowledgements
Public Health Service Grant DA07932 supported this research.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nation, J., Miller, D. & Bratton, G. Developmental Lead Exposure Alters the Stimulatory Properties of Cocaine at PND 30 and PND 90 in the Rat. Neuropsychopharmacol 23, 444–454 (2000). https://doi.org/10.1016/S0893-133X(00)00118-4
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1016/S0893-133X(00)00118-4
Keywords
This article is cited by
-
Maternal Lead Exposure Produces Long-Term Enhancement of Dopaminergic Reactivity in Rat Offspring
Neurochemical Research (2007)
-
Enhanced Acquisition of Cocaine Self-Administration in Rats Developmentally Exposed to Lead
Neuropsychopharmacology (2005)
-
Perinatal lead exposure and relapse to drug-seeking behavior in the rat: a cocaine reinstatement study
Psychopharmacology (2003)