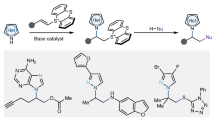
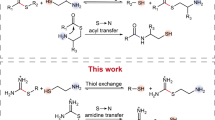
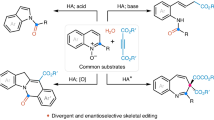
Abstract
A. A. Levine and A. G. Cole1 ozonized o-xylene and were able to isolate diacetyl from the reaction product, while methylglyoxal and glyoxal were isolated in the form of the osazones by means of p-nitrophenylhydrazine. This result is of great importance for the theory of the structure of the benzene nucleus. In their paper, Levine and Cole give no evidence about the yield of the substances isolated. Therefore we have reinvestigated this reaction using another analytical method. We have transformed the decomposition products of the ozonides into the corresponding oximes and we have worked out a method for separating the oximes quantitatively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Levine, A. A., and Cole, A. G., J. Amer. Chem. Soc., 54, 333 (1932).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WIBAUT, J., HAAYMAN, P. Ozonization of O-Xylene and the Structure of the Benzene Ring. Nature 144, 290 (1939). https://doi.org/10.1038/144290a0
Issue date:
DOI: https://doi.org/10.1038/144290a0