Abstract
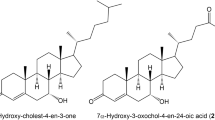
J. W. COOK1 pointed out that the sterols and bile acids contain in their molecules condensed carbon-ring systems to which are attached a side-chain in such a position that a new 6-membered ring can be formed so as to give the 1: 2-benzanthracene ring system without molecular rearrangement or group migration. The bile acids of the higher vertebrates are all mono-, di-, or tri-hydroxy derivatives of cholanic acid, which compound can be obtained in vitrofrom sterols by degradation, and the principal bile acids bear the names of lithocholic acid (3-hydroxy-cholanic acid), deoxycholic acid (3: 12-dihydroxy-cholanic acid) and cholic acid (3: 7: 12-trihydroxy-cholanic acid). J. W. Cook and G. A. D. Haslewood in 19342 showed that, in the formation of dehydronor-cholene from deoxycholic acid by the procedure of Wieland, such a ring-closure to the 1: 2-benzanthra-cene ring system had actually occurred. Dehydronor-cholene gave on dehyclrogenation the benzanthracene hydrocarbon methylcholanthrene, which was found to be strongly carcinogenic. Afterwards, Fieser and Newman3 showed that methylcholanthrene could be obtained also from cholic acid, which is the chief acid of human bile, and the parent hydrocarbon cholanthrene was synthesized by J. W. Cook, G. A. D. Haslewood and A. M. Robinson4 and shown to be carcinogenic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cook, J. W., and Kennaway, E. L., Chem. and Ind., 10, 521 (1932).
Cook, J. W., and Haslewood, G. A. D., J. Chem. Soc., 428 (1934).
Fieser, L. F., and Newman, M. S., J. Amer. Chem. Soc., 57, 961 (1935).
Cook, J. W., Haslewood, G. A. D., and Robinson, A. M., J. Chem. Soc., 667 (1935).
Andervont, H. B., Public Health Reports, 53, 1647 (1938).
Ghiron, V., Summary of Communications, 3rd International Cancer Congress, p. 1.16 (1939).
Shear, M. J., Amer. J. Cancer, 36, 211 (1939).
Parsons, L. D., NATURE, 144, 75 (1939).
Schabad, L., C.R. Soc. Biol., 124, 213 (1937).
Cook, J. W., NATURE, 145, 335 (1940).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
COOK, J., KENNAWAY, E. & KENNAWAY, N. Production of Tumours in Mice by Deoxycholic Acid. Nature 145, 627 (1940). https://doi.org/10.1038/145627a0
Issue date:
DOI: https://doi.org/10.1038/145627a0
This article is cited by
-
Bile acids and the gut microbiota: metabolic interactions and impacts on disease
Nature Reviews Microbiology (2023)
-
Gut microbiota-derived metabolites in CRC progression and causation
Journal of Cancer Research and Clinical Oncology (2021)
-
Concentration-dependent effects of sodium cholate and deoxycholate bile salts on breast cancer cells proliferation and survival
Molecular Biology Reports (2020)
-
Senescent hepatic stellate cells caused by deoxycholic acid modulates malignant behavior of hepatocellular carcinoma
Journal of Cancer Research and Clinical Oncology (2020)
-
Evaluating the structural complexity of isomeric bile acids with ion mobility spectrometry
Analytical and Bioanalytical Chemistry (2019)