Abstract
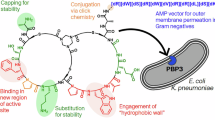
FLEMING1 noted that the growth of B. coli and a number of other bacteria belonging to the colityphoid group was not inhibited by penicillin. This observation has been confirmed. Further work has been done to find the cause of the resistance of these organisms to the action of penicillin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fleming, A., Brit. J. Exp. Path., 10, 226 (1929).
Booth, V. H., and Green, D. E., Biochem. J., 32, 855 (1938).
Chain, E., Florey, H. W., Gardner, A. D., Heatley, N. G., Jennings, M. A., Orr-Ewing, J., and Sanders, A. G., Lancet, 226 (1940).
MacLeod, C., J. Exp. Med., 72, 217 (1940).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ABRAHAM, E., CHAIN, E. An Enzyme from Bacteria able to Destroy Penicillin. Nature 146, 837 (1940). https://doi.org/10.1038/146837a0
Issue date:
DOI: https://doi.org/10.1038/146837a0
This article is cited by
-
Mycobacterium tuberculosis β-lactamase variant reduces sensitivity to ampicillin/avibactam in a zebrafish-Mycobacterium marinum model of tuberculosis
Scientific Reports (2023)
-
Exploiting the aggregation propensity of beta-lactamases to design inhibitors that induce enzyme misfolding
Nature Communications (2023)
-
Heterogeneity in M. tuberculosis β-lactamase inhibition by Sulbactam
Nature Communications (2023)
-
Antibiotic-resistant status and pathogenic clonal complex of canine Streptococcus canis-associated deep pyoderma
BMC Veterinary Research (2022)