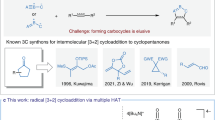
Abstract
WITH reference to Dr. Morton's suggestion regarding the role of vitamin A aldehyde (axerophthal) in the chemical changes involved in photo-reception1, it may be of interest to mention that we prepared this substance some eighteen months ago by Oppenauer oxidation of vitamin A alcohol with aluminium isopropoxide in the presence of acetaldehyde. The ultra-violet absorption spectrum of the aldehyde showed maxima at 350 and 368 mμ in cyclohexane and a band at 657 mμ in the antimony trichloride reaction, and was characterized by the formation of a 2:4-dinitrophenylhydrazone, m.p. 207–209°.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
NATURE, 153, 69 (1944).
Batty, Burawoy, Harper, Heilbron and Jones, J. Chem. Soc., 135 (1938).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HUNTER, R., HAWKINS, E. Vitamin A Aldehyde. Nature 153, 194 (1944). https://doi.org/10.1038/153194b0
Issue date:
DOI: https://doi.org/10.1038/153194b0
This article is cited by
-
Synthesis of Vitamin A Aldehyde
Nature (1947)
-
Preparation of Retinene in Vitro
Nature (1944)