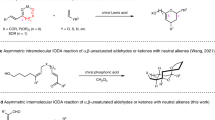
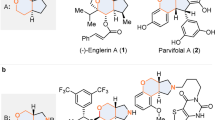
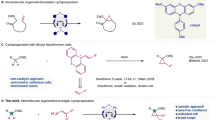
Abstract
IT has been suggested1 that 2:3-dihydrbfuran (I) on heating above 400° rearranges into cyclopropane aldehyde (II). By analogy, 2: 3-dihydropyran (III) should give cyclobutane aldehyde (IV). Experiments have shown, however, that, on heating, dihydropyran does not produce this aldehyde but splits by homogeneous reaction into acrolein and ethylene (V)2. Whether the cyclic aldehyde intervenes in the reaction or not must still remain an open question.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wilson, J. Chem. Soc., 59 (1945).
Wilson, Brit. P., 569625. Cf., also, Bremner and Jones, Brit. P., 573507.
Kline and Turkevich, J. Amer. Chem. Soc., 67, 498 (1945).
Paul, R., private communication.
Bremner and McNeil, Brit. P., 547334.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WILSON, C. Fission of 2: 3-Dihydropyran into Acrolein and Ethylene. Nature 157, 846 (1946). https://doi.org/10.1038/157846b0
Issue date:
DOI: https://doi.org/10.1038/157846b0