Abstract
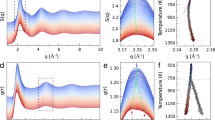
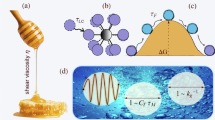
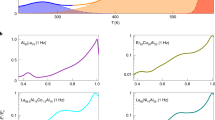
EXISTING theories of the viscosity of liquids fail to give a satisfactory account of the temperature variation of the viscosity of certain associated liquids. The X-ray diffraction patterns of liquids show that the inter-atomic distances vary about a mean; the variability increasing with increasing temperature. In addition, for the silicate glasses and for water, it is concluded that the structure is a random three-dimensional network of atomic bonds. This network is continuous throughout the liquid and in this sense glasses and water are associated liquids. In glasses the network is built up of Si—O bonds and in water of O—H bonds, the silicon atoms being surrounded by four oxygen atoms, and in water, the oxygen atoms by four hydrogen atoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bingham, "Fluidity and Plasticity", (McGraw-Hill, 1922). p. 339.
Ubbelohde, A. R., and Woodward, I., Proc. Roy. Soc., A, 185, 448 (1946).
Mott, N. F., and Gurney, R. W., "Electronic Processes in Ionic Crystals", (Oxford, 1940).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DOUGLAS, R. Viscosity of Associated Liquids. Nature 158, 415 (1946). https://doi.org/10.1038/158415a0
Issue date:
DOI: https://doi.org/10.1038/158415a0
This article is cited by
-
Annealing of Glass
Nature (1948)
-
Viscosity of Associated Liquids
Nature (1946)