Abstract
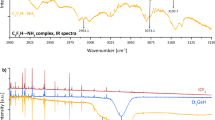
In 1941 Ketelaar1 published accurate measurements on the infra-red absorption and reflexion spectra of potassium hydrogen fluoride (KHF2). The two strong peaks of 1450 and 1222 cm.−1 were interpreted as a doubling of the fundamental asymmetric valence frequency v3 belonging to the vibration of a proton between two F− ions. The doubling was assumed to be caused by a double-minimum potential curve of the type occurring in the NH3 molecule. The splitting of the lowest vibrational level was estimated to be 25 cm.−1 and the distance between the two equilibrium positions 0.70 A.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ketelaar, J. A. A., Rec. Trav. Chim., 60, 523 (1941).
Dennison, D. M., and Uhlenbeck, G., Phys. Rev., 41, 313 (1932).
Van Vleek, J. H., "The Theory of Electric and Magnetic Susceptibilities" (Oxford, 1932).
Pitzer, K. S., and Westrum, E. F., J. Chem. Phys., 15, 526 (1947).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
POLDER, D. Nature of the Hydrogen Bond in Potassium Hydrogen Fluoride. Nature 160, 870–871 (1947). https://doi.org/10.1038/160870a0
Issue date:
DOI: https://doi.org/10.1038/160870a0