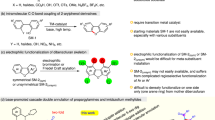
Abstract
WE wish to direct attention to the anomalous melting points (82–87° C.) quoted in the literature1–7 for diphenylene oxide. It is likely that these anomalies are due in part to the mode of formation, and we have examined the particular cases of the isolation of diphenylene oxide (a) from coal tar distillates, and (b) by the distillation of phenol over litharge2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hoffmeister, Ann., 159, 211 (m.p. 80–81° C.).
Gallewsky, Ann., 264, 189 (1891) (86–87° C.). ( Cullinane, J. Chem. Soc., 2268 (1930) (86–87° C.).)
Graebe and Ullmann, Ber., 29, 1876 (1896) (80–81° C.).
Cullinane, Morgan and Plummer, Rec. Trav. Chim., 56, 6 (83° C.).
Kruber, D.R.P., 491, 594 (84° C.).
Hale and Stoesser, U.S.P. 1,808,349 (82–83° C.).
"International Critical Tables", 1, 244 (87° C.).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WILLIAMS, A. Purity of Diphenylene Oxide (Dibenzofuran). Nature 162, 925 (1948). https://doi.org/10.1038/162925b0
Issue date:
DOI: https://doi.org/10.1038/162925b0