Abstract
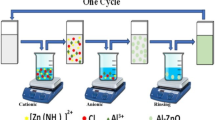
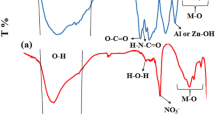
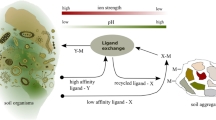
IN numerous communications, Feitknecht has described the preparation and structures of the basic salts and hydroxide of zinc. Particular reference is made in this communication to the so-called α-form of zinc hydroxide (Zn(OH)2), to which Feitknecht1 has ascribed a partially random structure, consisting of an assemblage of parallel layers, packed together without any further regularity. The analogy with the structures of the natural clay minerals mont-morillonite and halloysite suggested to one of us (D. M. C. M.) that it would be of interest to see if the α-hydroxide shows the same type of interlayer adsorption as these clays2. This appeared likely, because Feitknecht3 had shown that certain anionic dyestuffs are adsorbed on the α-zinc hydroxide, either by incorporating them in solution during the precipitation, or by shaking it with a solution of the dyestuff.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Feitknecht, W., Helv. Chim. Acta, 21, 766 (1938).
MacEwan, D. M. C., Trans. Farad. Soc., 44, 349 (1948).
Private communication.
Feitknecht, W., Helv. Chim. Acta, 26, 1911 (1943).
Feitknecht, W., Kolloid-Z., 92, 257 (1940).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MACEWAN, D., TALIB-UDDEEN, O. Adsorption Complexes of α-Zinc Hydroxide. Nature 163, 177–178 (1949). https://doi.org/10.1038/163177c0
Issue date:
DOI: https://doi.org/10.1038/163177c0