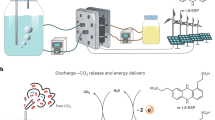
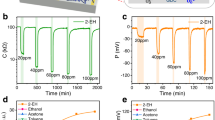
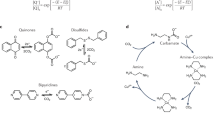
Abstract
A SUMMARY of the various recent views on the dissociation energy of carbon monoxide is given by Gaydon1 in his "Dissociation Energies", in which he eliminates all proposed values except 11·11 and 9·85 eV., and finally accepts the former as being the more probable. At about the same time, Kenty, Aicher, Noel, Paritsky and Paolino2 observed a new band system of molecular oxygen in a gas discharge containing carbon dioxide which suggested a lower limit for D (CO) of 9·4 eV. Since then, Hagstrum3 has defended his electron-impact value of 9·6 eV., Gëro4 through Valatin5 still retains faith in 6·9 eV., and Brewer, Gilles and Jenkins6 have made a determination of the heat of sublimation of graphite which indirectly supports 11·1 eV. Due to this confusion, which has now persisted for some time, the spectroscopic method of evaluating dissociation energies is in danger of being regarded with cynicism by those physical and organic chemists who urgently need this constant for carbon monoxide. The trouble lies in the lack of any dissociation continuum in the spectrum of carbon monoxide, and accordingly attention has been centred on predissociation phenomena, of which there seems no lack.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gaydon, A. G., "Dissociation Energies" (Chapman and Hall, Ltd., London, 1947).
Kenty, C., Aicher, J. O., Noel, E. B., Paritsky, A., and Paolino, V., Phys. Rev., 69, 36 (1946).
Hagstrum, H. D., Phys. Rev., 72, 947 (1947).
Gëro, L., J. Chem. Phys., 16, 1011 (1948).
Valatin, J. G., J. Chem. Phys., 16, 1018 (1948).
Brewer, L., Gilles, P. W., and Jenkins, F. A., J. Chem. Phys., 16, 797 (1948).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HOWELL, H. Dissociation Energy of Carbon Monoxide. Nature 163, 773 (1949). https://doi.org/10.1038/163773a0
Issue date:
DOI: https://doi.org/10.1038/163773a0