Abstract
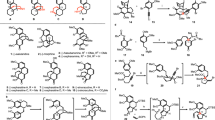
THE skeleton indicated is (I) and we first attempted an extension of the method whereby (II) had been synthesized in 19371. However, cyclization of 1-formamido-6: 7-dimethoxy-2-(2â-bromo-4â: 5â-dimethoxy)phenyl-1: 2: 3: 4-tetrahydronaphthalene could not be effected. R. D. Haworth2 was also interested in this problem and prepared 6: 7-dimethoxy-3-(3â: 4â-dimethoxy)phenacylphthalide (III), but was unable to add the elements of hydrocyanic acid to this substance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Richardson, Robinson and Seijo, J. Chem. Soc., 835 (1937).
Haworth, R. D., J. Chem. Soc., 1312 (1937).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BAILEY, A., ROBINSON, R. Synthesis of a Substance Containing the Chelerythrine-Sanguinarine Skeleton (C, N, O). Nature 164, 402 (1949). https://doi.org/10.1038/164402a0
Issue date:
DOI: https://doi.org/10.1038/164402a0