Abstract
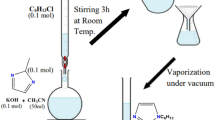
FOR the synthesis of esters from carboxylic acids and alcohols or phenols, it is customary to heat the reactants for several hours in the presence of a strong mineral acid catalyst, or else to proceed via the acid chloride or the acid anhydride. We now report a method of esterifleation which obviates the necessity of a two-stage process and, at the same time, enables a rapid direct reaction to occur between the acid and the hydroxy-compound under mild conditions. The new method, which entails the use of the anhydride of trifluoracetic acid, gives the purified esters in yields of 60–90 per cent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
STACEY, M., BOURNE, E., TATLOW, J. et al. A General Method of Esterification using Trifluoracetic Anhydride. Nature 164, 705 (1949). https://doi.org/10.1038/164705a0
Issue date:
DOI: https://doi.org/10.1038/164705a0