Abstract
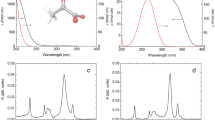
IT has been shown1 that weak acids are very often reduced polarographically in two steps. The more positive step belongs to the undissociated acid, the more negative to the acid ion. The total height of these two steps remains constant; but their ratio is changed in a characteristic way with the pH of the solution. If we plot this ratio against the pH, we obtain a curve which resembles very closely a dissociation curve. The pK of this dissociation curve is, however, generally shifted several pH units to higher values in comparison with the true acidity of the particular acid.
Similar content being viewed by others
Article PDF
References
Brdicka, R., and Wiesner, K., Coll., 12, 138 (1947); Chem. listy, 40, 66 (1946).
Wiesner, K., Chem. listy, 41, 6 (1947).
Brdicka, R., Coll., 12, 212 (1947).
Koutecky and Brdicka, Coll., 12, 338 (1947).
Britton, H. T. S., “Hydrogen Ions”, 225 (1932).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CLAIR, E., WIESNER, K. Dissociation Constants and Dissociation Velocities of Substituted Pyruvic Acids. Nature 165, 202 (1950). https://doi.org/10.1038/165202a0
Issue date:
DOI: https://doi.org/10.1038/165202a0