Abstract
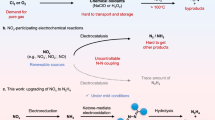
THE primary step in the decomposition of alkyl nitrates is considered to be the breaking of the O—N bond1,2,3. Phillips4 has shown that such decompositions are retarded by initially added nitrogen dioxide, and he has suggested that this primary step is an equilibrium reaction 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Appin, A., Chariton, J., and Todes, O., Acta Physicochimica Acta, U.R.S.S., 5, 655 (1936).
Bawn, C. E. H., and Adams, Trans. Farad. Soc., 45, 494 (1949).
Phillips, L., Nature, 160, 753 (1947).
Phillips, L. (private communication).
Smith, J. R. E., and Hinshelwood, C. N., Proc. Roy. Soc., A, 180, 237 (1942).
Steacie, E. W. R., “Atom and Free Radical Mechanisms” (Amer. Chem. Soc. Monograph No. 102).
Zeldovich, Y. B., and Shavlov, Yu. Kh., J. Phys. Chem., U.S.S.R., 20, 1359 (1946).
Norrish, R. G. W., and Foord, S. G., Proc. Roy. Soc., A, 152, 196 (1935)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
POLLARD, F., WYATT, R. & MARSHALL, H. Retarding (Inhibiting) and Sensitizing Effects of Nitrogen Dioxide in Alkyl Nitrate Decompositions. Nature 165, 564–565 (1950). https://doi.org/10.1038/165564b0
Issue date:
DOI: https://doi.org/10.1038/165564b0
This article is cited by
-
QSPR approach to the calculation of rate constants of homolysis of nitro compounds in different states of aggregation
Russian Chemical Bulletin (1995)