Abstract
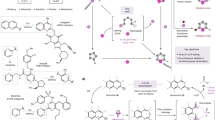
THE molecule of narcotine contains two asymmetric carbon atoms, IQ in the isoquinoline nucleus and P in the phthalide nucleus. The action of strong bases under suitable conditions racemizes P but leaves IQ unchanged1. Thus natural 1-α-narcotine is partly transformed into 1-β-narcotine; − IQ, − P ⇌ − IQ, + P.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Marshall, Pyman and Robinson, J. Chem. Soc., 1315 (1934).
Perkin, J. Chem. Soc., 118, 737 (1918).
Perkin and Robinson, J. Chem. Soc., 97, 305 (1910).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MIRZA, R., ROBINSON, R. Conversion of Hydrastine into Berberine, and an Instance of the Asymmetrical Quaternization of a Tertiary Base. Nature 166, 271–272 (1950). https://doi.org/10.1038/166271a0
Issue date:
DOI: https://doi.org/10.1038/166271a0
This article is cited by
-
Some generalizations relative to lithium aluminum hydride reactions
Experientia (1954)