Abstract
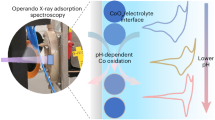
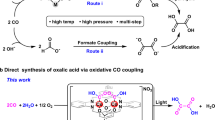
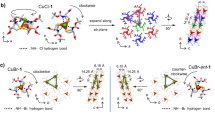
IT has been known for a long time that most amino-acids have the property of forming complexes with heavy metals, and it has now been shown1 that the cobaltous ion, Co++, combines reversibly with histidine, giving a tetra-co-ordinated compound, cobalto-dihistidine, which takes up oxygen reversibly to form a compound of octahedral configuration, oxy-bis-cobalto-dihistidine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Burk, D., Hearon, J., Caroline, L., and Shade, A. L., J. Biol. Chem., 165, 723 (1946).
Liquier-Milward, J., and Heath, J. C., Ann. Rep. Brit. Emp. Cancer Cam., 193 (1949).
Woodhouse, D. L., Arch. Biochem., 25, 347 (1950).
Ley, H., and Winkler, H., Ber., 42, 3894 (1909).
Bosworth Brown, G., Roll, P. M., and Plenth, A. A., Fed. Proc., 6 (2), 517 (1947).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LIQUIER-MILWARD, J. Evidence of a Complex Compound of Cobalt with a Purine Base (Adenine). Nature 167, 1068–1069 (1951). https://doi.org/10.1038/1671068b0
Issue date:
DOI: https://doi.org/10.1038/1671068b0
This article is cited by
-
Mutagenic effects of certain common metal toxicants on mammalian systems
Proceedings: Animal Sciences (1980)