Abstract
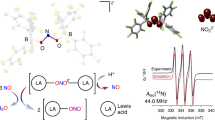
AS part of a programme of X-ray investigations of N—O bond-lengths and angles, and in particular for comparison of the bond-lengths in the positive (nitronium)1 and negative (nitrite) NO2 ions, the structure of sodium nitrite2 has been redetermined.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cox, E. G., Jeffrey, G. A., and Truter, M. R., Nature, 162, 259 (1948).
Ziegler, G. E., Phys. Rev., 38, 1040 (1931).
Cruickshank, D. W. J., Acta Cryst., 2, 65 (1949).
Grison, E., Eriks, K., and Vries, J. L., Acta Cryst., 3, 290 (1950).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
TRUTER, M. Dimensions of the Nitrite Ion. Nature 168, 344 (1951). https://doi.org/10.1038/168344b0
Issue date:
DOI: https://doi.org/10.1038/168344b0