Abstract
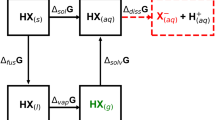
ALTHOUGH urea is an inexpensive laboratory chemical, its solvent properties do not appear to have been fully appreciated by chemists. Nevertheless, it may well prove to be one of the most versatile solvents known. Maintained at its melting point of 132° (conveniently done, if so desired, by refiuxing with xylene with which it is immiscible) its decomposition is very slow. It then combines the solvent properties of water with that of alcohols.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CLARK, R. Urea as a Solvent. Nature 168, 876 (1951). https://doi.org/10.1038/168876a0
Issue date:
DOI: https://doi.org/10.1038/168876a0