Abstract
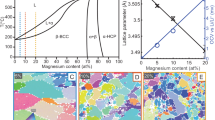
IN view of the possible commercial application of alloys of magnesium and lithium, the equilibrium diagram of these two metals has assumed practical as well as theoretical importance. The diagram is, in general, well established except for the solid solubility of magnesium in lithium.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Henry, O. H., and Cordiano, H. V., Trans. Amer. Inst. Min. Met. Eng., 111, 319 (1934).
Saldau, P., and Schamray, F. M., Z. anorg. Chem., 224, 388 (1935).
Grube, G., von Zeppelin, H., and Bumm, H., Z. Elektrochem., 40, 160 (1934).
Hume-Rothery, W., Raynor, G. V., and Butchers, E., J. Inst. Metals, 71, 589 (1945).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CATTERALL, J. Solubility of Magnesium in Lithium. Nature 169, 336 (1952). https://doi.org/10.1038/169336a0
Issue date:
DOI: https://doi.org/10.1038/169336a0
This article is cited by
-
Thermodynamic studies and the phase diagram of the Li-Mg system
Metallurgical and Materials Transactions A (1996)
-
The Li-Mg (Lithium-Magnesium) system
Bulletin of Alloy Phase Diagrams (1984)
-
Structure of Magnesium–Lithium β-Phase Alloys
Nature (1953)