Abstract
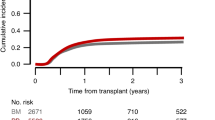
The study was designed to determine the toxicity, feasibility, and effectiveness of high-dose cyclophosphamide (6 g/m2), thiotepa (500 mg/m2) and carboplatin (800 mg/m2) (CTCb) with hematopoietic rescue as consolidation after standard-dose adjuvant chemotherapy treatment of primary high-risk breast cancer. From October 1991 to September 1994, 40 patients with stage II or III breast cancer involving 10 or more nodes were treated with CTCb after six cycles of adjuvant therapy with an anthracycline-containing regimen. Bone marrow (BM) was used as the source hematopoietic stem cell in the first 23 patients and G-CSF-mobilized peripheral blood progenitor cells (PBPC) in the other 17. No therapy-related deaths occurred, but three life-threatening complications were recorded which resolved: bilateral pulmonary hemorrhage, veno-occlusive disease of the liver and pulmonary thromboembolism. PBPC result in faster hemopoietic reconstitution with significantly lower transfusion requirements. With a median follow-up of 35 months (23–59) actuarial event-free survival for the study patients at 3 years is 72% (CI 95%: 66–81%). Even in patients over 50–60 years, CTCb is a relatively well tolerated regimen which appears, after a median follow-up of nearly 3 years, to decrease relapse frequency as compared with historical series, although a definite role of HDT in the treatment of high-risk primary breast cancer needs confirmation in prospective randomized trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tomás, J., Pérez-Carrión, R., Escudero, A. et al. Results of a pilot study of 40 patients using high-dose therapy with hematopoietic rescue after standard-dose adjuvant therapy for high-risk breast cancer. Bone Marrow Transplant 19, 331–336 (1997). https://doi.org/10.1038/sj.bmt.1700658
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/sj.bmt.1700658
Keywords
This article is cited by
-
Prognostic significance of the immunocytochemical detection of contaminating tumor cells (CTC) in apheresis products of patients with high-risk breast cancer treated with high-dose chemotherapy and stem cell transplantation
Bone Marrow Transplantation (2001)
-
High-dose chemotherapy and autologous stem cell transplantation as treatment for high-risk breast cancer
Cancer Chemotherapy and Pharmacology (1998)