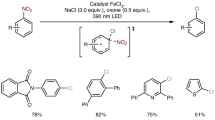
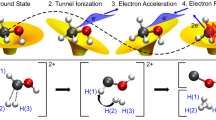
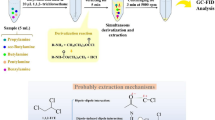
Abstract
THEORIES of gaseous unimolecular reactions have often been discussed1 ; but very few examples of processes occurring virtually completely by this mechanism have been used to compare theory and experiment. Thus the decompositions of azomethane, nitrous oxide and nitrogen pentoxide, which have been exhaustively examined from this point of view, are not now considered to be simple unimolecular reactions2. It is desirable, therefore, to have authenticated examples, and some evidence has already been presented that the homogeneous, first-order pyrolyses of ethyl, isopropyl and tert. butyl chlorides and of ethylidene dichloride are of this type3. This evidence is found mainly in comparisons with the obvious radical chain-decompositions of 1.2 dichloroethane and the tetrachloroethanes4. In contrast with the latter, the former show no induction periods, no inhibition by propylene, little sensitivity to oxygen and chlorine, and, in the case of ethyl chloride, there is reasonable agreement between the experimental activation energy and that calculated by the semi-empirical method.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Compare, for example, Chem. Rev., 10, 1 (1932), symposium on the kinetics of homogeneous reactions. Kassel, L. S., “Kinetics of Homogeneous Gas Reactions” (Chem. Catalogue Co., 1932). Hinshelwood, C. N., “Kinetics of Chemical Change” (Oxf. Univ. Press, 1940). Glasstone, S., Laidler, K. J., and Eyring, H., “Theory of Rate Processes” (McGraw-Hill, 1941).
See Hinshelwood, C. N., loc. cit., p. 124. Glasstone, G., Laidler, K. J., and Eyring, H., loc. cit., p. 296. Pease, R. N., “Equilibrium and Kinetics of Gas Reactions”, 129, 138 (Princeton Univ. Press, 1942). Ogg, R. A., J. Chem. Phys., 15, 337 (1947).
Barton, D. H. R., and Howlett, K. E., J. Chem. Soc., 165 (1949). Barton, D. H. R., and Onyon, P. F., Trans. Farad. Soc., 45, 725 (1949). Barton, D. H. R., and Head, A. J., Trans. Farad. Soc., 46, 114 (1950).
Barton, D. H. R., and Howlett, K. E., J. Chem. Soc., 155 (1949). Howlett, K. E., Nature, 165, 860 (1950) ; Trans. Farad. Soc., 48, 25 (1952). Barton, D. H. R., and Howlett, K. E., J. Chem. Soc., 2033 (1951).
Compare Hinshelwood, C. N., loc. cit., p. 81.
Computed from the viscosity data for ethane, by analogy with the variation in the chloromethanes ; see Partington, J. R., “Advanced Treatise on Physical Chemistry”, 860 (Longmans, 1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HOWLETT, K. Unimolecular Chlorohydrocarbon Decompositions. Nature 170, 168 (1952). https://doi.org/10.1038/170168a0
Issue date:
DOI: https://doi.org/10.1038/170168a0