Abstract
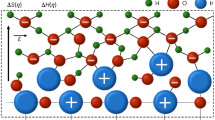
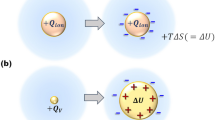
IT has been frequently observed that in some series of kindred reactions the activation energy of the process appears to be functionally related to the respective frequency factor A. For example, when one investigates a reaction taking place in various solvents, one often finds that E (the activation energy) and A change in the same direction. Such behaviour might indicate that the variation in the nature of the solvent affects the degree of solvation of the transition state, and consequently the activation energy and the entropy of activation (that is, A) in the same direction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Szwarc, J. Chem. Phys., 16, 128 (1948).
Szwarc and Leigh, Nature, 167, 486 (1951).
Steacie and Szwarc, J. Chem. Phys., 19, 1309 (1951).
Szwarc, Sehon and Ghosh, J. Chem. Phys., 18, 1142 (1950).
Sehon and Szwarc, Proc. Roy. Soc., A, 209, 97 (1951).
Ladacki, Leigh and Szwarc, Proc. Roy. Soc. (in the press).
Szwarc and Williams, J. Chem. Phys. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SZWARC, M., WILLIAMS, D. Relation between Activation Energy and Frequency Factor. Nature 170, 290 (1952). https://doi.org/10.1038/170290a0
Issue date:
DOI: https://doi.org/10.1038/170290a0
This article is cited by
-
Development and application of a tyrosinase-based time-temperature indicator (TTI) for determining the quality of turbot sashimi
Journal of Ocean University of China (2017)