Summary:
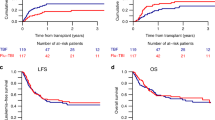
A total of 31 consecutive patients with hematologic malignancies who were considered poor candidates for TBI underwent allogeneic stem cell transplantation after conditioning with fludarabine and melphalan. A total of 25 matched sibling recipients received fludarabine 25 mg/m2 × 5 days and melphalan 70 mg/m2 × 2 days. For unrelated and haploidentical donor recipients, fludarabine was increased to 30 mg/m2 and ATG 30 mg/kg × 4 days was added. Graft-versus-host disease prophylaxis consisted of tacrolimus and mini methotrexate. All patients engrafted. Regimen-related toxicity was considerable and included mainly renal, hepatic and mucosal toxicity. There were seven regimen-related-deaths including two VOD, two pulmonary, one renal, one cardiac and one mucosal toxicity. One case of fatal pulmonary toxicity death could be attributed to pre-existing pulmonary damage. Progression-free survival at 12 months was 44% (90% CI: 30–58%) for recipients of HLA-identical sibling transplants and 33% (90% CI: 21–45%) for all patients. In conclusion, the fludarabine–melphalan regimen leads to consistent engraftment. The regimen-related toxicity is considerable and cannot be explained solely by patient selection. Cardiac toxicity is emerging as a unique toxicity of this regimen. Despite toxicity, fludarabine–melphalan has considerable activity and leads to durable remission in a proportion of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Giralt S, Thall PF, Khouri I et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001; 97: 631–637.
Slavin S, Nagler A, Naparstek E et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
McSweeney PA, Niederwieser D, Shizuru JA et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Devine SM, Sanborn R, Jessop E et al. Fludarabine and melphalan-based conditioning for patients with advanced hematological malignancies relapsing after a previous hematopoietic stem cell transplant. Bone Marrow Transplant 2001; 28: 557–562.
Przepiorka D, Khouri I, Ippoliti C et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after HLA-mismatched marrow or blood stem cell transplantation. Bone Marrow Transplant 1999; 24: 763–768.
Volpi I, Perruccio K, Tosti A et al. Postgrafting administration of granulocyte colony-stimulating factor impairs functional immune recovery in recipients of human leukocyte antigen haplotype-mismatched hematopoietic transplants. Blood 2001; 97: 2514–2521.
Przepiorka D, Ippoliti C, Panina A et al. Ganciclovir three times per week is not adequate to prevent cytomegalovirus reactivation after T-cell depleted marrow transplantation. Bone Marrow Transplant 1994; 13: 461–464.
Bearman SI, Appelbaum FR, Buckner CD et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
Przepiorka D, Weisdorf D, Martin P et al. Consensus conference on GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Papadopoulos EB, Carabasi MH, Castro-Malaspina H et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood 1998; 91: 1083–1090.
Soiffer RJ, Fairclough D, Robertson M et al. CD6-depleted allogeneic bone marrow transplantation for acute leukemia in first complete remission. Blood 1997; 89: 3039–3047.
Hansen JA, Gooley TA, Martin PJ et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med 1998; 338: 962–968.
Thomas ED . Bone marrow transplantation for malignant disease. J Clin Oncol 1983; 1: 517.
Bensinger WI, Martin PJ, Storer B et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 2001; 344: 175–181.
Champlin RE, Schmitz N, Horowitz MM et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT). Blood 2000; 95: 3702–3709.
Samuels BL, Britan JD . High-dose intravenous melphalan: a review. J Clin Oncol 1995; 13: 1786–1799.
Van Besien KW, Demuynck H, LeMaistre CF et al. High dose melphalan allows durable engraftment of allogeneic bone marrow. Bone Marrow Transplant 1995; 15: 321–323.
Giralt S, Estey E, Van Besien KW et al. Induction of graft-versus-leukemia without myeloablative therapy using allogeneic pbsc after purine analog containing regimens. Blood 1996; 88(Suppl. 1): 614a (Abstr.).
van Besien K, Devine S, Wickrema A et al. Fludarabine Melphalan is a suitable alternative to TBI-based conditioning in patients with advanced hematologic malignancies. Proceedings of 7th Annual Meeting of the European Hematology Association, 2002, pp. 135–142.
Sarosy G, Leyland-Jones B, Soochan P, Cheson BD . The systemic administration of intravenous melphalan. J Clin Oncol 1988; 6: 1768.
Ho VT, Soiffer RJ . The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 2001; 98: 3192–3204.
Chao N . Pharmacology and use of immunosuppressive agents after hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ (eds). Hematopoietic Cell Trans-plantation. Blackwell Sciences, Inc.: Malden, MA, 1999, pp 176–185.
Morgenstern GR, Powles R, Robinson B, Mc Elwain TJ . Cyclosporin interaction with ketoconazole and melphalan. Lancet 1982; 2: 1342.
Ritchie DS, Seymour JF, Roberts AW et al. Acute left ventricular failure following melphalan and fludarabine conditioning. Bone Marrow Transplant 2001; 28: 101–103.
Martino R, Caballero MD, Canals C et al. Allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning: results of a prospective multicentre study. Br J Haematol 2001; 115: 653–659.
Ferrara JLM, Antin JH . The pathophysiology of graft vs host disease. In: Thomas ED, Blume KG, Forman SJ (eds). Hematopoietic Cell Transplantation. Blackwell Sciences: Malden, MA, 1999, pp 305–315.
Przepiorka D, Saliba R, Anderlini P et al. Chronic graft vs host disease after allogeneic stem cell transplantation. Blood 2001; 98: 1695–1700.
Folz RJ . Mechanisms of lung injury after bone marrow transplantation. Am J Respir Cell Mol Biol 1999; 20: 1097–1099.
Kottaridis PD, Milligan DW, Chopra R et al. In vivo CAMPATH-1 H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000; 96: 2419–2425.
Robinson SP, Mackinnon S, Goldstone A et al. Higher than expected transplant-related mortality and relapse following non-myeloablative stem cell transplantation for lymphoma adversely affects survival. Blood 2000; 96(Suppl. 1): (Abstr. 2380). p. 554a.
Yunis JJ, Brunning RD, Howe RB, Lobell M . High-resolution chromosomes as an independent prognostic indicator in adult acute nonlymphocytic leukemia. N Engl J Med 1984; 311: 812.
Bacigalupo A . Second EBMT Workshop on reduced intensity allogeneic hemopoietic stem cell transplants (RI-HSCT). Bone Marrow Transplant 2002; 29: 191–195.
Author information
Authors and Affiliations
Additional information
Supported in part by a grant from Berlex Pharmaceuticals
Rights and permissions
About this article
Cite this article
van Besien, K., Devine, S., Wickrema, A. et al. Regimen-related toxicity after fludarabine–melphalan conditioning: a prospective study of 31 patients with hematologic malignancies. Bone Marrow Transplant 32, 471–476 (2003). https://doi.org/10.1038/sj.bmt.1704166
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.bmt.1704166
Keywords
This article is cited by
-
Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation
Journal of Thrombosis and Thrombolysis (2021)
-
Cardiac Complications in the Adult Bone Marrow Transplant Patient
Current Oncology Reports (2019)
-
Fludarabine and neurotoxicity in engineered T-cell therapy
Gene Therapy (2018)
-
Cardiovascular Complications of Hematopoietic Stem Cell Transplantation
Current Treatment Options in Cardiovascular Medicine (2016)
-
Comparison of reduced conditionings combining fludarabine with melphalan or 3-day busulfan in patients allografted for myeloid neoplasms
International Journal of Hematology (2014)