Abstract
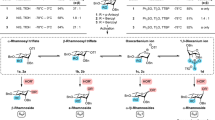
RECENT investigations1 of the behaviour of the Dische reagent2 towards various deoxysugars and their glycosides have revealed that a coloration is obtained with a number of such compounds, and that the reagent cannot be regarded as specific for 2-deoxy-ribose. Colorimetric evidence based on the Disehe test thus does not preclude the occurrence of deoxysugars other than 2-deoxy ribose3 in the deoxy-pentose nucleic acid structure. As an extension to the investigations on the known 2-deoxyribose4 and 3-deoxyribose5 (otherwise known as 3-deoxyxylose), we have synthesized 4-deoxy-L-ribose, with a view to its characterization and the study of its behaviour towards colorimetric reagents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Allerton, R., Overend, W. G., and Stacey, M., J. Chem. Soc., 255 (1952).
Deriaz, R. E., et al., J. Chem. Soc., 1222 (1949).
Levene, P. A., and Jacobs, W. A., J. Biol. Chem., 12, 411 (1912). Levene, P. A., J. Biol. Chem., 48, 119 (1921). Kent, P. W., Nature, 166, 442 (1950).
Meisenheimer, J., and Jung, H., Ber., 60, 1462 (1927). Deriaz, R. E., et al., J. Chem. Soc., 1879 (1949).
Kent, P. W., Stacey, M., and Wiggins, L. F., J. Chem. Soc., 1232 (1949).
Peat, S., “Adv. Carbohyd. Chem.”, 2, 49 (Acad. Press, N.Y., 1946).
Hockett, R., and McClenahan, W. S., J. Amer. Chem. Soc., 61, 1667 (1939).
Jones, J. N. K., et al., J. Chem. Soc., 930 (1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WARD, P., KENT, P. 4-Deoxy-L-Ribose. Nature 170, 936 (1952). https://doi.org/10.1038/170936a0
Issue date:
DOI: https://doi.org/10.1038/170936a0